Summary
We quantified midline kinematics with synchronized electromyograms (emgs) from the red and white muscles on both sides of bluegill sunfish (Lepomis macrochirus) during escape behaviors which were elicited from fish both at a standstill and during steady speed swimming. Analyses of variance determined whether or not kinematic and emg variables differed significantly between muscle fiber types, among longitudinal positions, and between swimming versus standstill trials.
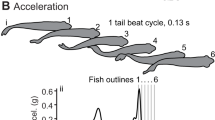
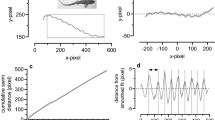
At a given longitudinal location, both the red and white muscle were usually activated synchronously during both stages of the escape behavior. Stage 1 emg onsets were synchronous; however, the mean durations of stage 1 emgs showed a significant increase posteriorly from about 11 to 15 ms. Stage 2 emgs had significant posterior propagation, but the duration of the stage 2 emgs was constant (17 ms). Posterior emgs from both stages occurred during lengthening of the contractile tissue (as indicated by lateral bending). Steady swimming activity was confined to red muscle bursts which were propagated posteriorly and had significant posterior decrease in duration from about 50% to 37% of a cycle. Fish performed escape responses during all phases of the steady swimming motor pattern. All kinematic events were propagated posteriorly. Furthermore, no distinct kinematic event corresponded to the time intervals of the stage 1 and 2 emgs. The rate of propagation of kinematic events was always slower than that of the muscle activity. The phase relationship between lateral displacement and lateral bending also changed along the length of the fish. Escape responses performed during swimming averaged smaller amplitudes of stage 2 posterior lateral displacement; however, most other kinematic and emg variables did not vary significantly between these two treatments.
Similar content being viewed by others
Abbreviations
- A:
-
angle of lateral flexion (bending) of midline at a single point in time
- A1, A2:
-
change in A from T0 to T1 and from T1 to T2
- AMX:
-
maximal lateral flexion concave towards the side of the stage 1 emg
- AMXR:
-
equals AMX minus A at T0
- AT1, AT2:
-
lateral flexion at T1 and T2
- DUR1, DUR2:
-
durations of stage 1 and stage 2 emgs
- emg:
-
electromyogram
- ON2:
-
onset time of stage 2 emg
- RELDUR:
-
relative duration of steady swimming emg
- T0, T1, T2 :
-
times of stage 1 emg onset, latest stage 1 emg offset and latest stage 2 emg offset standardized such that T0 = 0
- TAMX, TAMN, TYMX:
-
times of maximal lateral flexion, no lateral flexion and maximum lateral displacement
- Y1, Y2:
-
amounts of lateral displacement from T0 to T1 and from T1 to T2
- YMXR:
-
relative amount of lateral displacement from T0 to TYMX
References
Blight AR (1976) Undulatory swimming with and without waves of contraction. Nature 264:352–354
Blight AR (1977) The muscular control of vertebrate swimming movements. Biol Rev 52:181–218
Bone Q (1966) On the function of the two types of myotomal muscle fiber in elasmobranch fish. J Mar Biol Assoc UK 46:321–349
Bone Q (1978) Locomotor muscle. In: Hoar WS, Randall DJ (eds) Fish physiology. Vol 7. Locomotion. Academic Press, New York, pp 361–424
Covell JW, Smith M, Harper DG, Blake RW (1991) Skeletal muscle deformation in the lateral muscle of the intact rainbow trout Oncorhynchus mykiss during fast start maneuvers. J Exp Biol 156:453–466
Eaton RC, Emberley DS (1991) How stimulus direction determines the trajectory of the Mauthner-initiated escape response in a teleost fish. J Exp Biol 161:469–487
Eaton RC, Hackett JT (1984) The role of the Mauthner cell in fast-starts involving escape in teleost fishes. In: Eaton RC (ed) Neural mechanisms of startle behavior. Plenum Press, New York, pp 213–266
Eaton RC, Bombardieri RA, Meyer DL (1977) The Mauthner-initiated startle response in teleost fish. J Exp Biol 66:65–81
Eaton RC, Lavender WA, Wieland CM (1981) Identification of Mauthner-initiated response patterns in goldfish: evidence from simultaneous cinematography and electrophysiology. J Comp Physiol 144:521–531
Eaton RC, Lavender WA, Wieland CM (1982) Alternative neural pathways initiate fast-start responses following lesions of the Mauthner neuron in goldfish. J Comp Physiol 145:485–496
Eaton RC, DiDomenico R, Nissanov J (1988) Flexible body dynamics of the goldfish C-start: implications for reticulospinal command mechanisms. J Neurosci 8:2758–2768
Eaton RC, DiDomenico R, Nissanov J (1991) The role of the Mauthner cell in sensorimotor integration by the brainstem escape network. Brain Behav Evol 37:272–285
Fetcho JR (1986) The organization of the motoneurons innervating the axial musculature of vertebrates. I. Goldfish (Carassius auratus) and mudpuppies (Necturus maculosus). J Comp Neurol 249:521–550
Fetcho JR (1991) Spinal network of the Mauthner cell. Brain Behav Evol 37:298–316
Fetcho JR (1992) A single action potential in a reticulospinal neuron can reset the fictive swimming rhythm in goldfish. Soc Neurosci Abstr No. 140.1
Foreman MB, Eaton RC (1993) The direction change concept for reticulospinal control of goldfish escape. J Neurosci 13:4101–4113
Frolich LM, Biewener AA (1992) Kinematic and electromyographic analysis of the functional role of the body axis during terrestrial and aquatic locomotion in the salamander Ambystoma tigrinum. J Exp Biol 162:107–130
Gray J (1968) Animal locomotion. Weidenfield and Nicolson, London
Grillner S, Kashin S (1976) On the generation and performance of swimming in fish. In: Herman R, Grillner S, Stein, Stuart D (eds) Neural control of locomotion. Plenum Press, New York, pp 181–202
Harper DG, Blake RW (1990) Fast-start performance of rainbow trout Salmo gairdneri and Northern pike Esox lucius. J Exp Biol 150:321–342
Hudson RCL (1973) On the function of the white muscles in teleosts at intermediate speeds. J Exp Biol 58:509–522
Jayne BC (1988) Muscular mechanisms of snake locomotion: An electromyographic study of the lateral undulation of the Florida banded water snake (Nerodia fasciata) and the yellow rat snake (Elaphe obsoleta). J Morphol 197:159–181
Johnston IA (1991) Muscle action during locomotion: a comparative perspective. J Exp Biol 160:167–185
Lee MT, Olin AM (1992) Interaction between Mauthner neurons and the swimming pattern generator in Xenopus tadpoles. Soc Neurosci Abstr No. 139.16
Rayner MD Keenan MJ (1967) Role of red and white muscles in the swimming of the skipjack tuna. Nature 214:392–393
Rome LC, Sosnicki A A (1991) Myofilament overlap in swimming carp II. Sarcomere length changes during swimming. Am J Physiol 260 (Cell Physiol 29):C289-C296
Rome LC, Loughna, PT, Goldspink G (1984) Muscle fiber recruitment as a function of swim speed and muscle temperature in carp. Am J Physiol 247:R272-R279
Rome LC, Choi IH, Lutz G, Sosnicki A (1992a) The influence of temperature on muscle function in the fast swimming scup I. Shortening velocity and muscle recruitment during swimming.J Exp Biol 163:259–279
Rome LC, Sosnicki A, Choi IH (1992b) The influence of temperature on muscle function in the fast swimming scup II. The mechanics of red muscle. J Exp Biol 162:281–295
Van Leeuwen JL, Lankheet MJM, Akster HA, Osse JWM (1990) Function of red axial muscles of carp (Cyprinus carpio): recruitment and normalized power output during swimming in different modes. J Zool Lond 220:123–145
Wardle CS (1975) Limit of fish swimming speed. Nature 255:725–727
Webb PW (1975) Hydrodynamics and energetics of fish propulsion. Bull Fish Res Board Can 190
Webb PW (1978) Fast start performance and body form in seven species of teleost fish. J Exp Biol 74:211–226
Webb PW, Skadsen JM (1980) Strike tactics of Esox. Can J Zool 58:1462–1469
Weihs D (1973) The mechanism of rapid starting of slender fish. Biorheology 10:343–350
Williams TL, Grillner S, Smoljaninov W, Wallen P, Kashin S, Rossignol S (1989) Locomotion in lamprey and trout: the relative timing of activation and movement. J Exp Biol 143:559–566
Zar JH (1984) Biostatistical analysis. Prentice Hall, Englewood Cliffs, New Jersey
Zottoli SJ (1977) Correlation of the startle reflex and Mauthner cell auditory responses in unrestrained goldfish. J Exp Biol 66:243–254
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jayne, B.C., Lauder, G.V. Red and white muscle activity and kinematics of the escape response of the bluegill sunfish during swimming. J Comp Physiol A 173, 495–508 (1993). https://doi.org/10.1007/BF00193522
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00193522