Summary
-
1.
70% of primary afferent fibres in the bobtail lizard were irregularly spontaneously active, with rates between 0.1 and 123.7 spikes/s (mean of 31.2 spikes/s). The rate distribution is bimodal. Mid-frequency fibres with lower thresholds tend to have higher spontaneous rates.
-
2.
About one third of all fibres show a more prominent mode in their inter-spike interval histogram than is to be expected from a quasi-Poisson distribution. Preferred intervals in the spontaneous discharge patterns were not normally seen; this is presumably due to the lack of exclusive innervation of hair cells.
-
3.
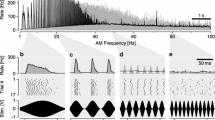
Primary fibres mostly responded to sound with a discharge rate increase. The form of the peri-stimulustime histogram varies between fibres; three different response types are described. Fibres of low characteristic frequency (CF up to 0.65 kHz) show a characteristic change in their response pattern with stimulation frequency; it is suggested that primary suppression plays an important role in shaping the very phasic response to tones at the fibres' upper frequency range. The responses of higher-CF fibres (CF 0.55–4 kHz) are independent of stimulation frequency. About one third of them shows a primary-like discharge pattern. The majority, however, responds with a chopper-like discharge pattern and there is evidence that this discharge, as in mammalian cochlear-nucleus stellate cells, originates from temporal summation.
-
4.
The systematically-varying response pattern typical for low-CF fibres introduces special difficulties in defining the extent of the excitatory tuning curve and of the two-tone rate suppression area. The influence of threshold criteria on the overlap seen between both curves is discussed.
Similar content being viewed by others
Abbreviations
- CF :
-
characteristic frequency
- PSTH :
-
peri-stimulus time histogram
- TIH :
-
time-interval histogram
- TTRS :
-
two-tone rate suppression
References
Abbas PJ, Sachs MB (1976) Two-tone suppression in auditoryerve fibers: Extension of a stimulus-response relationship. J Acoust Soc Am 59:112–122
Anastasio TJ, Correia MJ, Perachio AA (1985) Spontaneous and driven responses of semicircular canal primary afferents in the unanaesthetized pigeon. J Neurophysiol 54:335–347
Arthur RM, Pfeiffer RR, Suga N (1971) Properties of ‘two-tone inhibition’ in primary auditory neurones. J Physiol 212:593–609
Ashmore JF (1982) Spontaneous membrane potential oscillations in frog saccular hair cells. J Physiol 336:24P
Capranica RR, Moffat AJM (1980) Nonlinear properties of the peripheral auditory system of anurans. In: Popper AN, Fay RR (eds) Comparative studies of hearing in vertebrates. Springer, Berlin Heidelberg New York, pp 139–165
Coombs S, Fay RR (1987) Response dynamics of goldfish saccular fibers: Effects of stimulus frequency and intensity on fibers with different tuning, sensitivity and spontaneous activity. J Acoust Soc Am 81:1025–1035
Crawford AC, Fettiplace R (1980) The frequency selectivity of auditory nerve fibres and hair cells in the cochlea of the turtle. J Physiol 306:79–125
Düring M von, Karduck A, Richter AG (1974) The fine structure of the inner ear in Caiman crocodilus. Z Anat Entwickl Gesch 145:41–65
Eatock RA (1978) Auditory nerve fibre activity in the tokay gecko. MSc thesis, Biology Dept of McGill Univ, Montreal
Eatock RA, Weiss TF (1986) Relation of discharge rate to sound pressure level for cochlear nerve fibers in the alligator lizard. Abstr 9th midwinter research meeting of the ARO, Clearwater Beach, Florida
Eatock RA, Manley GA, Pawson L (1981) Auditory nerve fibre activity in the tokay gecko: I. Implications for cochlear processing. J Comp Physiol 142:203–218
Fettiplace R (1987) Electrical tuning of hair cells in the inner ear. Trend Neurosci 10:421–425
Frezza WA (1976) Spontaneous activity in the auditory nerve of the alligator lizard. B Sc thesis, Dept Electr Eng, Mass Inst Technology
Geisler CD (1981) A model for discharge patterns of primary auditory-nerve fibers. Brain Res 212:198–201
Gross NB, Anderson DJ (1976) Single unit responses recorded from the first order neuron of the pigeon auditory system. Brain Res 101:209–222
Hill KG, Mo J, Stange G (1989) Excitation and suppression of primary auditory fibres in the pigeon. Hearing Res 39:37–48
Holton T (1980) Relations between frequency selectivity and twotone rate suppression in lizard cochlear-nerve fibers. Hearing Res 2:21–38
Holton T, Weiss TF (1983) Receptor potentials of lizard cochlear hair cells with free-standing stereocilia in response to tones. J Physiol 345:205–240
Klinke R, Pause M (1980) Discharge properties of primary auditory fibres in Caiman crocodilus; comparisons and contrasts to the mammalian auditory nerve. Exp Brain Res 38:137–150
Kiang NYS, Watanabe T, Thomas EC, Clark LF (1965) Discharge patterns of single fibers in the cat's auditory nerve. MIT Press, Cambridge, Massachusetts
Köppl C, Manley GA (1990) Peripheral auditory processing in the bobtail lizard Tiliqua rugosa. II. Tonotopic organization and innervation pattern of the basilar papilla. J Comp Physiol A 167:101–112
Köppl C, Manley GA, Johnstone BM (1990) Peripheral auditory processing in the bobtail lizard Tiliqua rugosa. V. Seasonal effects of anaesthesia. J Comp Physiol A 167:139–144
Konishi M (1970) Comparative neurophysiological studies of hearing and vocalizations in songbirds. Z Vergl Physiol 66:257–272
Konishi M, Sullivan WE, Takahashi T (1985) The owl's cochlear nuclei process different sound localization cues. J Acoust Soc Am 78:360–364
Lewis ER (1988) Tuning in the bullfrog ear. Biophys J 53:441–447
Liberman MC (1978) Auditory-nerve response from cats raised in a low-noise chamber. J Acoust Soc Am 63:442–455
Liff HJ, Goldstein MH (1970) Peripheral inhibition in auditory fibers in the frog. J Acoust Soc Am 47:1538–1547
Manley GA (1977) Response patterns and peripheral origin of auditory nerve fibres in the monitor lizard, Varanus bengalensis. J Comp Physiol 118:249–260
Manley GA (1979) Preferred intervals in the spontaneous activity of primary auditory neurones. Naturwissenschaften 66:582
Manley GA (1981) A review of the auditory physiology of the reptiles. Progr Sens Physiol 2:49–134
Manley GA (1990) Peripheral hearing mechanisms in reptiles and birds. Springer, Berlin Heidelberg New York
Manley GA, Gleich O (1984) Avian primary auditory neurones: the relationship between characteristic frequency and preferred intervals. Naturwissenschaften 71:592–594
Manley GA, Gleich O, Leppelsack H-J, Oeckinghaus H (1985) Activity patterns of cochlear ganglion neurones in the starling. J Comp Physiol A 157:161–181
Manley GA, Gleich O, Kaiser A, Brix J (1989) Functional differentiation of sensory cells in the avian auditory periphery. J Comp Physiol A 164:289–296
Manley GA, Köppl C, Johnstone BM (1990a) Peripheral auditory processing in the bobtail lizard Tiliqua rugosa. I. Frequency tuning of auditory-nerve fibres. J Comp Physiol A 167:89–99
Manley GA, Yates GK, Köppl C, Johnstone BM (1990b) Peripheral auditory processing in the bobtail lizard Tiliqua rugosa. IV. Phase locking of auditory-nerve fibres. J Comp Physiol A 167:129–138
Megela AL, Capranica RR (1981) Response patterns to tone bursts in peripheral auditory system of anurans. J Neurophysiol 46:465–478
Miller MR (1975) The cochlear nuclei of lizards. J Comp Neurol 159:375–406
Miller MR (1978) Further scanning electron microscope studies of lizard auditory papillae. J Morphol 156:381–418
Miller MR (1985) Quantitative studies of auditory hair cells and nerves in lizards. J Comp Neurol 232:1–24
Miller MR, Beck J (1988) Auditory hair cell innervational patterns in lizards. J Comp Neurol 271:604–628
Miller MR, Beck J (1990) Further serial TEM studies of auditory hair cell innervation in lizards and in a snake. Am J Anat in press
Mulroy MJ (1986) Patterns of afferent synaptic contacts in the alligator lizard's cochlea. J Comp Neurol 248:263–271
Oertel D (1985) Use of brain slices in the study of the auditory system: spatial and temporal summation of synaptic inputs in cells in the anteroventral cochlear nucleus of the mouse. J Acoust Soc Am 78:328–333
Patuzzi R, Robertson DR (1988) Tuning in the mammalian cochlea. Phys Rev 68:1009–1082
Pfeiffer RR (1966) Classification of response patterns of spike discharges for units in the cochlear nucleus; tone-burst stimulation. Exp Brain Res 1:220–235
Rhode WS (1985) The use of intracellular techniques in the study of the cochlear nucleus. J Acoust Soc Am 78:320–327
Rose C, Weiss TF (1988) Frequency dependence of synchronization of cochlear nerve fibres in the alligator lizard: Evidence for a cochlear origin of timing and non-timing neural pathways. Hearing Res 33:151–166
Runhaar G (1988) The surface morphology of the avian tectorial membrane. Hearing Res 37:179–188
Sachs MB, Young ED, Lewis RH (1974) Discharge patterns of single fibers in the pigeon auditory nerve. Brain Res 70:431–447
Sachs MB, Woolf NK, Sinnott JM (1980) Response properties of neurons in the avian auditory system: comparisons with mammalian homologues and consideration of the neural encoding of complex stimuli. In: Popper AN, Fay RR (eds) Comparative studies of hearing in vertebrates. Springer, Berlin Heidelberg New York, pp 323–353
Schermuly L, Klinke R (1985) Change of characteristic frequencies of pigeon primary auditory afferents with temperature. J Comp Physiol A 156:209–211
Smolders JWT, Klinke R (1984) Effects of temperature on the properties of primary auditory fibres of the spectacled caiman, Caiman crocodilus (L.). J Comp Physiol A 155:19–30
Sneary MG (1988a) Auditory receptor of the red-eared turtle: I. General ultrastructure. J Comp Neurol 276:573–587
Sneary MG (1988b) Auditory receptor of the red-eared turtle: II. Afferent and efferent synapses and innervation pattern. J Comp Neurol 276:588–606
Sullivan WE (1985) Classification of response patterns in cochlear nucleus of barn owl: correlation with functional response properties. J Neurophysiol 53:201–216
Temchin AN (1988) Discharge patterns of single fibres in the pigeon's auditory nerve. J Comp Physiol A 163:99–115
Teresi PV (1985) Hair cell innervation patterns in the papilla basilaris of the fence lizard Sceloporus occidentalis. Dissertation, Univ of California, San Francisco
Turner RG (1980) Physiology and bioacoustics in reptiles. In: Popper AN, Fay RR (eds) Comparative studies of hearing in vertebrates. Springer, Berlin Heidelberg New York, pp 205–237
Walsh BT, Miller JB, Gesek RR, Kiang NYS (1972) Spontaneous activity in the eighth cranial nerve of the cat. Intern J Neurosci 3:221–236
Weiss TF (1966) A model of the peripheral auditory apparatus. Kybernetik 3:153–175
Weiss TF, Mulroy MJ, Turner RG, Pike CL (1976) Tuning of single fibres in the cochlear nerve of the alligator lizard: relation to receptor morphology. Brain Res 115:71–90
Yagi T, Ueno H (1988) Behavior of primary horizontal canal neurons in alert and anesthetized guinea pigs. Exp Neurol 101:356–365
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Köppl, C., Manley, G.A. Peripheral auditory processing in the bobtail lizard Tiliqua rugosa . J Comp Physiol A 167, 113–127 (1990). https://doi.org/10.1007/BF00192411
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00192411