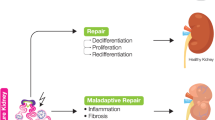
Conclusion
The mechanisms that regulate regeneration of kidney epithelial cells after acute tubular necrosis are poorly understood. Repair of the nephron can take place in the adverse systemic metabolic setting caused by failure of renal function. This clinical observation suggests that factors released at the site of the tubular insult can mediate repair. Studies carried out in this and other laboratories show that kidney epithelial cells can release and respond to polypeptide growth factors which may thereby contribute to renal regeneration by autocrine and paracrine mechanisms. Specific growth factors secreted by cells and deposited in the tubular basement membrane prior to injury may subsequently participate in nephron repair. In addition, adenine nucleotides released from injured or dying cells at the injury site or provided exogenously could stimulate surviving renal epithelial cells at the edges of the wound to migrate along the basement membrane to rapidly reepithelialize the nephron and subsequently initiate mitogenesis to replace cells lost by necrosis.
The nephrotoxic effect of many agents used in cancer chemotherapy and the older age of patients undergoing complicated surgical procedures has increased the incidence of ARF, whereas the mortality rate has not changed since the early 1950s [22]. Thus there is considerable need for innovative therapeutic strategies. An important goal of future research efforts is to identify new growth factors that facilitate migration, differentiation, and proliferation of renal epithelial cells at sites of tubular necrosis. Isolation and use of these agents in combination with dialysis and nutritional support could speed renal regeneration and thereby improve the outcome in patients with this condition.
Similar content being viewed by others
Abbreviations
- ARF:
-
acute renal failure
- ECM:
-
extracellular matrix
- EGF:
-
epidermal growth factor
- FGF:
-
fibroblast growth factor
- IGF:
-
insulin-like growth factor
- MGSA:
-
melanocyte growth-stimulating activity
- PDGF:
-
platelet-derived growth factor
- IGF:
-
transforming growth factor
References
Bade EG, Feindler S (1988) Liver epithelial cell migration induced by epidermal growth factor or transforming growth factor alpha is associated with changes in the gene expression of secreted proteins.In Vitro Cell Dev Biol 24:149–154
Bellas RE, Bendori R, Farmer SR (1991) Epidermal growth factor activation of vinculin and β1-integrin gene transcription in quiescent Swiss 3T3 cells. J Biol Chem 266:12008–12014
Berridge MJ (1987) Inositol trisphoshate and diacylglycerol: two interacting second messengers. Annu Rev Biochem 56:159–193
Cuppage FE, Cunningham N, Tate AL (1969) Nucleic acid synthesis in the regenerating nephron following injury with mercuric chloride. Lab Invest 21:449–457
Dinarello CA (1988) Interleukin-1. Rev Infect Dis 6:51–95
Fine LG, Holley RW, Nasri H, Bade-Dezfooly B (1985) BSC-1 growth inhibitor transforms a mitogenic stimulus into a hypertrophic stimulus for renal proximal tubular cells: relationship to Na+/H+ antiport activity. Proc Natl Acad Sci USA 82:6163–6166
Humes HD, Cielinski DA, Coimbra TM, Messana JM, Galvao C (1989) Epidermal growth factor enhances renal tubular cell regeneration in postischemic acute renal failure. J Clin Invest 84:1757–1761
Kartha S, Toback FG (1985) Purine nucleotides stimulate DNA synthesis in kidney epithelial cells in culture. Am J Physiol 249:F967-F972
Kartha S, Toback FG (1992) Adenine nucleotides stimulate migration in wounded cultures of kidney epithelial cells. J Clin Invest 90:288–292
Kartha S, Sukhatme VP, Toback FG (1987) ADP activates protooncogene expression in renal epithelial cells. Am J Physiol 252:F1175-F1179
Kartha S, Bradham DM, Gotendorst GR, Toback FG (1988) Kidney epithelial cells express the c-sis proto-oncogene and secrete PDGF-like protein: evidence for a paracrine mechanism. Am J Physiol 255:F800-F806
Massagué J (1990) Transforming growth factor-α. J Biol Chem 265:21393–21396
Mendley SR, Toback FG (1989) Autocrine and paracrine regulation of kidney epithelial cell growth. Annu Rev Physiol 51:33–50
Miller SB, Martin DR, Kissane J, Hammerman MR (1992) Insulin-like growth factor I accelerates recovery from acute tubular necrosis in the rat. Proc Natl Acad Sci USA 89:11876–11880
Nagaike M, Hirao S, Tajima H, Noji S, Taniguchi T, Matsumoto K, Nakamura T (1991) Renotropic functions of hepatocyte growth factor in renal regeneration after unilateral nephrectomy. J Biol Chem 266:22781–22784
Rall LB, Scott J, Bell GI, Crawford RJ, Penshow JD, Niall HD, Coghlan JP (1985) Mouse prepro-epidermal growth factor synthesis by the kidney and other tissues. Nature 313:228–231
Rangnekar VV, Waheed S, Davies TJ, Toback FG, Rangnekar VM (1991) Antimitogenic and mitogenic actions of interleukin-1 in diverse cell types are associated with induction of gro gene expression. J Biol Chem 266:2415–2422
Roberts AB, Flanders KC, Heine UI, Jakowlew S, Kondaiah P, Kim SJ, Sporn MB (1990) Transforming growth factor-β: multifunctional regulator of differentiation and development. Philos Trans R Soc Lond Biol 327:145–154
Siegel NJ, Gaudio KM (1988) Amino acids and adenine nucleotides in acute renal failure. In: Brenner BM, Lazarus JM (eds) Acute renal failure, 2nd ed. Churchill Livingstone, New York, pp 857–873
Sukhatme VP, Kartha S, Toback FG, Taub R, Hoover RV, Tsai-Morris C-H (1987) A novel early growth response gene rapidly induced by fibroblast, epithelial cell and lymphocyte mitogens. Oncogene Res 1:343–355
Toback FG (1985) Control of renal regeneration after acute tubular necrosis. In: Robinson RR (ed) Proceedings of the IXth International Congress of Nephrology, vol 1. Springer, Berlin Heidelberg New York, pp 748–763
Toback FG (1992) Regeneration after acute tubular necrosis. Kidney Int 41:226–246
Walsh-Reitz MM, Toback FG (1984) Kidney epithelial cell growth is stimulated by lowering extracellular potassium concentration. Am J Physiol 247:C14-C19
Walsh-Reitz MM, Gluck SL, Waack S, Toback FG (1986) Lowering extracellular Na+ concentration releases autocrine growth factors from renal epithelial cells. Proc Natl Acad Sci USA 83:4764–4768
Yarden Y, Ullrich A (1988) Molecular analysis of signal transduction by growth factors. Biochemistry 27:3113–3119
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Toback, F.G., Kartha, S. & Walsh-Reitz, M.M. Regeneration of kidney tubular epithelial cells. Clin Investig 71, 861–866 (1993). https://doi.org/10.1007/BF00190339
Issue Date:
DOI: https://doi.org/10.1007/BF00190339