Abstract
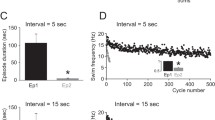
The effects of serotonin on the electrical properties of swim-gating neurons (cell 204) were examined in leech (Hirudo medicinalis) nerve cords. Exposure to serotonin decreased the threshold current required to elicit swim episodes by prolonged depolarization of an individual cell 204 in isolated nerve cords. This effect was correlated with a more rapid depolarization and an increased impulse frequency of cell 204 in the first second of stimulation. In normal leech saline, brief depolarizing current pulses (1 s) injected into cell 204 failed to elicit swim episodes. Following exposure to serotonin, however, identical pulses consistently evoked swim episodes. Thus, serotonin appears to transform cell 204 from a gating to a trigger cell.
Serotonin had little effect on the steady-state currentvoltage relation of cell 204. However, serotonin altered the membrane potential trajectories in response to injected current pulses and increased the amplitude of rebound responses occurring at the offset of current pulses. These changes suggest that serotonin modulates one or more voltage dependent conductances in cell 204, resulting in a more rapid depolarization and greater firing rate in response to injected currents. Thus, modulation of intrinsic ionic conductances in cell 204 may account in part for the increased probability of swimming behavior induced by serotonin in intact leeches.
Similar content being viewed by others
Abbreviations
- AHP:
-
afterhyperpolarizing potential
- DCC:
-
discontinuous current clamp
- DP:
-
dorsal posterior nerve
- G2:
-
segmental ganglion 2
- PIR:
-
postinhibitory rebound
- RMP:
-
resting membrane potential
References
Angstadt JD, Calabrese RL (1989) A hyperpolarization-activated inward current in heart interneurons of the medicinal leech. J Neurosci 9:2846–2857
Arbas EA, Calabrese RL (1987) Ionic conductances underlying the activity of interneurons that control heartbeat in the medicinal leech. J Neurosci 7:3945–3952
Brodfuehrer PD, Friesen WO (1986a) Initiation of swimming activity by trigger neurons in the leech subesophageal ganglion. II. Role of segmental swim-initiating interneurons. J Comp Physiol A 159:503–510
Brodfuehrer PD, Friesen WO (1986b) From stimulation to undulation: A neuronal pathway for the control of swimming in the leech. Science 234:1002–1004
Debski EA, Friesen WO (1986) Role of central interneurons in habituation of swimming activity in the medicinal leech. J Neurophysiol 55:977–944
Friesen WO (1985) Neuronal control of leech swimming movements: interactions between cell 60 and previously described oscillator neurons. J Comp Physiol A 156:231–242
Friesen WO (1986) Synaptic interactions between inhibitory leech motor neurons in isolated nerve cords and in cell culture. Soc Neurosci Abstr 12:359
Friesen WO (1989) Neural control of leech swimming movements. In: Jacklet JW (ed) Neuronal and cellular oscillators. Marcel Dekker Inc, New York, pp 269–316
Friesen WO, Poon M, Stent GS (1978) Neuronal control of swimming in the medicinal leech: IV. Identification of a network of oscillatory interneurones. J Exp Biol 75:25–43
Giachetti A, Shore PA (1978) The reserpine receptor. Life Sci 23:89–92
Hashemzadeh-Gargari H, Friesen WO (1989) Modulation of swimming activity in the medicinal leech by serotonin and octopamine. Comp Biochem Physiol 94C: 295–302
Johnson RG Jr (1988) Accumulation of biological amines into chromaffin granules: a model for hormones and neurotransmitter transport. Physiol Rev 68:232–307
Kristan WB, Calabrese RL (1976) Rhythmic swimming activity in neurones of the isolated nerve cord of the leech. J Exp Biol 65:643–668
Kristan WB, Stent GS, Ort CA (1974) Neuronal control of swimming in the medicinal leech. I. Dynamics of the swimming rhythm. J Comp Physiol 94:97–119
Lent CM (1985) Serotonergic modulation of the feeding behavior of the medicinal leech. Brain Res Bull 14:643–655
Macagno ER (1980) Number and distribution of neurons in the leech segmental ganglion. J Comp Neurol 190:283–302
Muller KJ, Nicholls JG, Stent GS (eds) (1981) Neurobiology of the leech. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY
Mangan PM, Friesen WO (1990) Serotonin enhances synaptic fatigue in neuronal circuits of the leech. Soc Neurosci Abstr 16:1130
O'Gara BA, Chae H, Latham LB, Friesen WO (1991) Modification of leech behavior patterns by reserpine-induced amine depletion. J Neurosci 11:96–110
Pearce RA, Friesen WO (1984) Intersegmental coordination of leech swimming: comparison of in situ and isolated nerve cord activity with body wall movement. Brain Res 299:363–366
Sawyer RY (1981) Leech biology and behavior. In: Muller KJ, Nicholls JG, Stent GS (eds) Neurobiology of the leech. Cold Spring Harbor Laboratory, New York, pp 7–26
Shore PA (1962) Release of serotonin and catecholamines by drugs. Pharmacol Rev 14:531–550
Slotkin TA (1974) Reserpine. In: Simpson LL, Curtis DR (eds) Neuropoisons: their pathophysiological actions, vol. 2, Poisons of plant origin. Plenum, New York, pp 1–60
Truman JW, Weeks JC (1985) Activation of neuronal circuits by circulating hormones in insects. In: Selverston AI (ed) Model neural networks and behavior. Plenum, New York, pp 381–399
Weeks JC (1981) Neuronal basis of leech swimming: separation of swim initiation, pattern generation, and intersegmental coordination by selective lesions. J Neurophysiol 45(4): 698–723
Weeks JC (1982a) Segmental specialization of a leech swim-initiating interneuron, cell 205. J Neurosci 2:972–985
Weeks JC (1982b) Synaptic basis of swim initiation in the leech. I. Connections of a swim-initiating neuron (cell 204) with motor neurons and pattern-generating “oscillator” neurons. J Comp Physiol 148:253–263
Weeks JC, Kristan WB (1978) Initiation, maintenance and modulation of swimming in the medicinal leech by the activity of a single neurone. J Exp Biol 77:71–88
Willard AL (1981) Effects of serotonin on the generation of the motor program for swimming by the medicinal leech. J Neurosci 1:936–944
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Angstadt, J.D., Friesen, W.O. Modulation of swimming behavior in the medicinal leech. J Comp Physiol A 172, 223–234 (1993). https://doi.org/10.1007/BF00189398
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00189398