Abstract
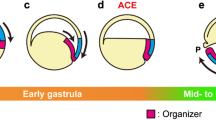
The isolated upper marginal zone from the initial stage of Cynops gastrulation is not yet determined to form the dorsal axis mesoderm: notochord and muscle. In this experiment, we will indicate where the dorsal mesoderm-inducing activity is localized in the very early gastrula, and what is an important event for specification of the dorsal axis mesoderm during gastrulation. Recombination experiments showed that dorsal mesoderm-inducing activity was localized definitively in the endodermal epithelium (EE) of the lower marginal zone, with a dorso-ventral gradient; and the EE itself differentiated into endodermal tissues, mainly pharyngeal endoderm. Nevertheless, when dorsal EE alone was transplanted into the ventral region, a secondary axis with dorsal mesoderm was barely formed. However, when dorsal EE was transplanted with the bottle cells which by themselves were incapable of mesoderm induction, a second axis with well-developed dorsal mesoderm was observed. When the animal half with the lower marginal zone was rotated 180° and recombined with the vegetal half, most of the rotated embryos formed only one dorsal axis at the primary blastopore side. The present results suggest that there are at least two essential processes in dorsal axis formation: mesoderm induction of the upper marginal zone by endodermal epithelium of the lower marginal zone, and dorsalization of the upper dorsal marginal zone evoked during involution.
Similar content being viewed by others
References
Cho KWY, Blumberg B, Steinbeisser H, De Robertis FM (1991) Molecular nature of Spemann's organizer: the role of the Xenopus homeobox gene goosecoid. Cell 67:1111–1120
Christian JL, Olson DJ, Moon RT (1992) Xwnt-8 modifies the character of mesoderm induced by bFGF in isolated Xenopus ectoderm. EMBO J 11:33–41
Dale I, Slack JMW (1987) Regional specification within the mesoderm of early embryos of Xenopus laevis. Development 100:279–295
Elison RP, Kao KR (1989) The location of dorsal information in frog early development. Dev Growth Differ 31:423–430
Forman D, Slack MW (1980) Determination and cellular commitment in the embryonic amphibian mesoderm. Nature 286: 492–494
Fujisue M, Kobayakawa Y, Yamana K (1993) Occurrence of dorsal axis-inducing activity around the vegetal pole of an uncleaved Xenopus egg and displacement to the equatorial region by cortical rotation. Development 118:163–170
Gerhart J, Danilchik M, Doniach T, Roberts S, Rowning B, Stewart R (1989) Cortical rotation of the Xenopus egg: consequences for the anteroposterior pattern of embryonic dorsal development. Development 107 (Suppl):37–52
Gerhart JC, Doniach T, Stewart R (1991) Organizing the Xenopus organizer. In: Keller R, Clark W Jr, Griffin F. Gastrulation: movements, patterns and molecules. (eds) Plenum, New York
Grunz H (1990) Homoeogenetic neural inducing activity of the presumptive neural plate of Xenopus laevis. Dev Growth Differ 32:583–589
Grunz H (1993) The dorsalization of Spemann's organizer takes place during gastrulation in Xenopus laevis embryos. Dev Growth Differ 35:25–32
Hainski AM, Moody SA (1992) Xenopus maternal RNAs from a dorsal animal blastomere induce a secondary axis in host embryos. Development 116:347–355
Hama T, Tsujimura H, Kaneda T, Takata K, Ohara A (1985) Inductive capacities for the dorsal mesoderm of the dorsal marginal zone and pharyngeal endoderm in the very early gastrula of the newt, and presumptive pharyngeal endoderm as an initiator of the organization center. Dev Growth Differ 27:419–433
Holtfreter-Ban H (1965) Differentiation capacities of Spemann's organizer investigated in explants of diminishing size. Ph D thesis, University of Rochester
Kaneda T (1980) Studies on the formation and state of determination of the trunk organizer in the newt, Cynops pyrrhogaster II. Inductive effect from the underlying cranial archenteron roof. Dev Growth Differ 22:841–849
Kaneda T (1981) Studies on the formation and state of determination of the trunk organizer in the newt, Cynops pyrrhogaster III. Tangential induction in the dorsal marginal zone. Dev Growth Differ 23:553–564
Keller R, Shih J, Domingo C (1992) The patterning and functioning of protrusive activity during convergence and extension of the Xenopus organizer. Development (Suppl):81–91
Kimelman D, Christian JL, Moon RT (1992) Synergistic principles of development: overlapping patterning systems in Xenopus mesoderm induction. Development 116:1–9
Lettice LA, Slack JMW (1993) Properties of the dorsalizing signal in gastrula of Xenopus laevis. Development 117:263–271
Nakamura O, Takasaki H, Ishihara M (1971) Formation of the organizer from combinations of presumptive ectoderm and endoderm. Proc Jpn Acad 47:313–318
Nieuwkoop PD (1969) The formation of the mesoderm in urodelean amphibians. I. Induction by the endoderm. Wilhelm Roux' Arch Ertwicklungsmech Org 162:341–373
Okada Y, Ichikawa M (1947) Normal table of Triturus (Cynops) pyrrhogaster. Jpn J Exp Morphol 3:1–6
Shih J, Keller R (1992) The epithelium of the dorsal marginal zone of Xenopus has organizer properties. Development 116:887–899
Smith JC, Slack JMW (1983) Dorsalization and neural induction: properties of the organizer in Xenopus laevis. J Embryol Exp Morphol 78:299–317
Smith WC, Harland RM (1991) Injected Xwnt-8 acts early in Xenopus embryos to promote formation of a vegetal dorsalizing center. Cell 67:753–766
Spemann H, Mangold H (1924) Über Induktion von Embryonalanlagen durch Implantation artfremder Organisatoren. Wilhelm Roux' Arch Entwicklungsmech Org 100:599–638
Suzuki AS, Miki K (1983) Cellular basis of neuralization of induced neuroectoderm in amphibian embryogenesis: changes of cell shape, cell size, and cytodifferentiation of the neuroectoderm after neural induction. Dev Growth Differ 25:289–297
Suzuki AS, Mifune Y, Kaneda T (1984) Germ layer interactions in pattern formation of amphibian mesoderm during primary embryonic induction. Dev Growth Differ 26:81–94
Yamada T (1940) Beeinflussung der Differenzierungsleistung des isolierten Mesoderms von Molchkeimen durch zugefügtes Chorda- und Neural-Material. Okajimas Folia Anat Jpn 19:131–197
Yamada T (1949) In: New Biology (in Japanese). Nihon Kagakusha, Kyoto, pp 85–116
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yamamoto, Y., Suzuki, A.S. Two essential processes in the formation of a dorsal axis during gastrulation of Cynops embryo. Roux's Arch Dev Biol 204, 11–19 (1994). https://doi.org/10.1007/BF00189063
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00189063