Summary
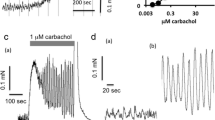
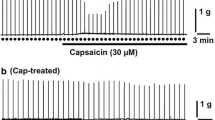
The effects of nicotine receptor agonists on the release of [3H]acetylcholine from the phrenic nerve, the small intestine and the trachea were investigated to characterize neuronal nicotine receptors within the peripheral nervous system. Contraction of the indirectly-stimulated hemidiaphragm was recorded to investigate desensitization of the postsynaptic muscular nicotine receptors. Nicotine, cytisine, 1,1-dimethyl-4-phenylpiperazinium and 2-(4-aminophenyl)-ethyl-trimethyl-ammoniumiodide caused a concentration-dependent (0.1–30 μM) increase in evoked [3H]acetylcholine release from the phrenic nerve, whereby bell-shaped concentration-response curves were obtained. The rank order of decreasing potency was: nicotine > cytisine > 1,1-dimethyl-4-phenylpiperazinium > 2-(4-aminophenyl)-ethyl-trimethyl-ammoniumiodide. The presynaptic effects of nicotine depended strongly on the exposure time: facilitation occurred after a short 20 s exposure and inhibition after a 3 min exposure, whereas nicotine no longer affected evoked [3H]acetylcholine release after a 15 min exposure. Pre-exposure (40 min) of the phrenic nerve to 0.3 μM nicotine prevented any subsequent modulatory effect of a high nicotine concentration. In contrast, the contraction of the indirectly-stimulated hemidiaphragm remained unaffected in the presence of 0.3–30 μM nicotine, but a concentration of 1 mM nicotine abolished skeletal muscle contraction. Nicotine (10 μM) produced a substantial release of [3H]acetylcholine in the small intestine but not in the isolated trachea. The present experiments show presynaptic nicotine receptors at the phrenic nerve, which, under appropriate conditions, can mediate facilitation of evoked transmitter release. These neuronal receptors appear more sensitive to desensitizing conditions than the postsynaptic muscular nicotine receptors. Nicotine also mediates a transient release of acetylcholine in the myenteric plexus but not in the trachea, and as a consequence, applied nicotine preferentially activates smooth muscle activity in the intestine.
Similar content being viewed by others
Abbreviations
- DMPP:
-
1,1-dimethyl-4-phenylpiperazinium
- PAPETA:
-
2-(4-aminophenyl)-ethyl-trimethylammoniumiodiderine of [3H]acetylcholine release
References
Beani L, Bianchi C, Nilsson L, Nordberg A, Romanelli L, Sivilotti L (1985) The effects of nicotine and cytisine on [3H]acetylcholine release from cortical slices of guineapig brain. Naunyn Schmiedebergs Arch Pharmacol 331:293–296.
Bowman WC (1990) Pharmacology of neuromuscular transmission, 2nd edn. Wright, London.
Bowman WC, Gibb AJ, Harvey AL, Marshall IG (1986) Prejunctional actions of cholinoceptor agonists and antagonists, and of anticholinesterase drugs. In: Kharkevich DA (ed) New neuromuscular blocking agents. (Handbook of Experimental Pharmacology, vol 79, p 141) Springer, Berlin Heidelberg New York.
Chesselet MF (1984) Presynaptic regulation of neurotransmitter release in the brain. Neuroscience 12:347–375.
Clarke PBS (1987) Recent progress in identifying nicotinic cholinoceptors in mammalian brain. Trends Pharmacol Sci 8:32–35.
Deneris ES, Connolly J, Rogers SW, Duvoisin R (1991) Pharmacological and functional diversity of neuronal nicotinic acetylcholine receptors. Trends Pharmacol Sci 12:34–40.
Dzieniszewski P, Kilbinger H (1978) Muscarinic modulation of acetylcholine release evoked by dimethylphenylpiperazinium and high potassium from guinea-pig myenteric plexus. Eur J Pharmacol 50:385–391.
Domino EF (1973) Neuropsychopharmacology of nicotine and tobacco smoking. In: Dunn WL (ed) Smoking behavior: motives and incentives. Winston/Wiley, New York p 5.
Ferry CB (1988) The origin of the anticholinesterase-induced repetitive activity of the phrenic nerve-diaphragm preparation of the rat in vitro. Br J Pharmacol 94:169–179.
Gilbert DG (1979) Paradoxial tranquilizing and emotion-reducing effects of nicotine. Psychol Bull 86:643–661.
Heffron PF, Hobbiger F (1979) Relationship between inhibition of acetylcholinesterase and response of the rat phrenic nerve-diaphragm preparation to indirect stimulation at higher frequencies. Br J Pharmacol 66:323–329.
Katz B, Thesleff S (1957) A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol (Lond) 138:63–80.
Kilbinger H, Wessler I (1980) Inhibition by acetylcholine of the stimulation-evoked release of [3H]acetylcholine from the guinea-pig myenteric plexus. Neuroscience 5:1331–1340.
Löffelholz K (1970) Autoinhibition of nicotinic release of noradrenaline from postganglionic sympathetic nerves. Naunyn Schmiedebergs Arch Pharmacol 267:49–63.
Löffelholz K (1978) In: Paton DM (ed) The release of catecholamines from adrenergic neurons. Pergamon Press, Oxford New York p 275.
Loiacono RE, Mitchelson FJ (1990) Effect of nicotine and tacrine on acetylcholine release from rat cerebral cortical slices. Naunyn Schmiedebergs Arch Pharmacol 342:31–35.
Lundberg JM, Martling CR, Lundblad L (1988) Cigarette smoke-induced irritation in the airways in relation to peptide-containing, capsaicin-sensitive sensory neurons. Klin Wochenschr 66 [Suppl XI]:151–160.
Masland RL, Wigton RS (1940) Nerve activity accompanying fasciculation produced by prostigmin. J Neurophysiol 3:269–275.
Riker WF (1975) Prejunctional effects of neuromuscular blocking and facilitatory drugs. In: Katz RL (ed) Muscle relaxants. (Monographs in Anesthesiology, vol 3, p 59) North-Holland Publishing Co, Amsterdam.
Stolerman IP, Fink R, Jarvik ME (1973) Acute and chronic tolerance to nicotine measured by activity in rats. Psychopharmacology 30:329–342.
Vizi ES, Somogyi GT, Nagashima H, Duncalf D, Chaudhry IA, Kobayashi O, Goldiner PL, Foldes FF (1987) d-Tubocurarine and pancuronium inhibit evoked release of acetylcholine from the mouse hemidiaphragm preparation. Br J Anaesth 59:226–231.
Wallenstein S, Zucker CL, Fleiss JI (1980) Some statistical methods useful in circulation research. Circ Res 47:1–9.
Wessler I (1989) Control of transmitter release from the motor nerve by presynaptic nicotinic and muscarinic autoreceptors. Trends Pharmacol Sci 10:110–114.
Wessler I, Kilbinger H (1986) Release of [3H]acetylcholine from a modified rat phrenic nerve-hemidiaphragm preparation. Naunyn Schmiedebergs Arch Pharmacol 334:357–364.
Wessler I, Steinlein O (1987) Differential release of [3H]ace-tylcholine from the rat phrenic nerve-hemidiaphragm preparation by electrical nerve stimulation and by high potassium. Neuroscience 22:289–299.
Wessler I, Werhand J (1990) Evaluation by reverse phase HPLC of [3H]acetylcholine release evoked from the myenteric plexus of the rat. Naunyn Schmiedebergs Arch Pharmacol 341:510–516.
Wessler I, Halank M, Rasbach J, Kilbinger H (1986) Presynaptic nicotine receptors mediating a positive feed-back on transmitter release from the rat phrenic nerve. Naunyn Schmiedebergs Arch Pharmacol 334:365–372.
Wessler I, Apel Ch, Hillen U (1988) Increase by tubocurine of rine of [3]acetylcholine release from the rat phremic nerve desensitization fo nicotine receptors. Eur J Pharmacol 151: 139–142.
Wessler I, Hellwig D, Racké K (1990) Epithelium-derived inhibition of [3H]acetylcholine release from the isolated guinea-pig trachea. Naunyn Schmiedebergs Arch Pharmacol 342:387–393.
Wessler I, Klein A, Pohan D (1991) Release of [3H]acetylcholine from the isolated trachea evoked by preganglionic nerve stimulation: comparison with transmural stimulation. Naunyn Schmiedebergs Arch Pharmacol 343 [Suppl.]:R88.
Wonnacott S, Irons J, Rapier C, Thorne B, Lunt GG (1989) Presynaptic modulation of transmitter release by nicotine receptors. In: Nordberg A, Fuxe K, Holmstedt B, Sundwall A (eds) Progressum Brain Research, vol 79 p 157. Elsevier Sci, Amsterdam.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wessler, I., Apel, C., Garmsen, M. et al. Effects of nicotine receptor agonists on acetylcholine release from the isolated motor nerve, small intestine and trachea of rats and guinea-pigs. Clin Investig 70, 182–189 (1992). https://doi.org/10.1007/BF00184649
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF00184649