Summary
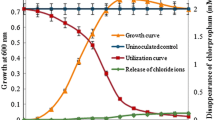
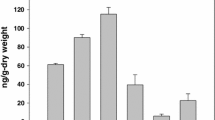
A bacterium capable of utilizing biphenyl as the sole source of carbon was isolated from soil and identified as a Micrococcus species. The organism also utilized 4-chlorobiphenyl and several other aromatic compounds as growth substrates. 2,3-Dihydroxybiphenyl and benzoic acid were identified as intermediates by physico-chemical methods. The bacterium degraded biphenyl to 2,3-dihydroxybiphenyl followed by its meta-ring cleavage to yield 2-hydroxy-6-oxo-6-phenylhexa-2,4-dienoic acid, which was then hydrolysed to give benzoic acid. Benzoate was further metabolised via a catechol meta-cleavage pathway by a Micrococcus sp.
Similar content being viewed by others
References
Buchanan RE, Gibbons NE (eds) (1974) Bergey's manual of determinative bacteriology, 8th edn. The Williams & Wilkins Co, Baltimore
Catelani D, Colombi A (1974) Metabolism of biphenyl structure and physiochemical properties of 2-hydroxy-6-oxo-6-phenyl-hexa-2,4-dienoic acid the meta-cleavage product from 2,3-dihydroxybiphenyl by Pseudomonas putida. Biochem J 143:431–434
Catelani D, Sorlini C, Treccani V (1971) The metabolism of biphenyl by Pseudomonas putida. Experientia 27:1173–1174
Catelani D, Colombi A, Sorlini C, Treccani V (1973) Metabolism of biphenyl. 2-Hydroxy-6-oxo-6-phenylhexa-2,4-dienoate: the meta-cleavage product from 2,3-dihydroxybiphenyl by Pseudomonas putida. Biochem J 134:1063–1066
Durham DR, McNamee CG, Stewart DB (1984) Dissimilation of aromatic compounds in Rhodotorula graminis: biochemical characterisation of pleiotrophically negative mutants. J Bacteriol 160:771–777
Gibson DT, Roberts RL, Wells MC, Kobal VM (1973) Oxidation of biphenyl by a Beijerinckia species. Biochem Biophys Res Commun 50:211–219
Hayaishi O, Katagiri M, Rothberg S (1957) Studies on oxygenases. Pyrocatechase. J Biol Chem 229:905–920
Ishigooka H, Yoshida Y, Omori T, Minoda Y (1986) Enzymatic dioxygenation of biphenyl-2,3-diol and 3-isopropylacatechol. Agric Biol Chem 50:1045–1046
Kojima Y, Itada N, Hayaishi O (1961) Metapyrocatechase: a new catechol cleaving enzyme. J Biol Chem 236:2223–2228
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Lunt DO, Evans WC (1970) The microbial metabolism of biphenyl. Biochem J 118:54P
Nozaki M (1970) Metapyrocatechase (Pseudomonas). Methods Enzymol 17A:522–525
Omori T, Sugimura K, Ishigooka H, Minoda Y (1986) Purification and some properties of a 2-Hydroxy-6-oxo-6-phenylexa-2,4-dienoic acid hydrolysing enzyme from Pseudomonas cruciviae S93B1 involved in the degradation of biphenyl. Agric Biol Chem 50:931–937
Risebrough RW, Lappe B de (1972) Accumulation of polychlorinated biphenyls in ecosystems. Environ Health Perspect 1:39–49
Seubert W (1960) Determination of isoprenoid compound by microorganisms I. Isolation and characterization of an isoprenoid-degrading bacterium, Pseudomonas citronellolis, new species. J Bacteriol 79:426–434
Smith MR, Ratledge C (1989) Catabolism of biphenyl by Pseudomonas sp NCIB 10643 and Nocardia sp NCIB 10503. Appl Microbiol Biotechnol 30:395–401
Starovoitov II, Selfonov SA, Nefedova MYU, Adain VM (1986) Catabolism of diphenyl by Pseudomonas putida strain BS893 containing biodegradation plasmid pBS241 (English translation). Microbiology 54:726–730
Yamaguchi M, Yamauchi T, Fujisawa H (1975) Mechanism of double hydroxylation. I. Evidence for participation of NADH-cytochrome C reductase in the reaction of benzoate 1,2-dioxygenase (benzoate hydroxylase). Biochem Biophys Res Commun 67:264–271
Author information
Authors and Affiliations
Additional information
Correspondence to: H. Z. Ninnekar
Rights and permissions
About this article
Cite this article
Bevinakatti, B.G., Ninnekar, H.Z. Degradation of biphenyl by a Micrococcus species. Appl Microbiol Biotechnol 38, 273–275 (1992). https://doi.org/10.1007/BF00174482
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00174482