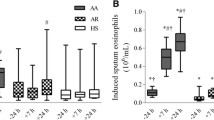
Abstract
Based on a growing body of evidence, allergic as well as intrinsic bronchial asthma have recently been defined as chronic persistent inflammatory disorders. Agreement has been reached that asthma can no longer be equated with bronchospasm only, and that the absence of reversibility of airflow obstruction does not exclude bronchial asthma. Bronchial hyperreactivity, on the other hand, although common to the vast majority of asthmatics, is not specific for bronchial asthma and provocation tests to measure bronchial hyperreactivity are not suited for routine monitoring of bronchial asthma. The clinical features of asthma are related to cellular as well as to soluble parameters of bronchial inflammation. Therefore, means of assessing and monitoring asthmatic inflammation have been investigated. Since eosinophils, T lymphocytes, mast cells, macrophages, neutrophils, epithelial cells, and structural cells, as well as various proinflammatory mediators and proteins, have been implicated in the pathogenesis of bronchial asthma, it has been anticipated that several of these cells or mediators might be either diagnostic of bronchial asthma or could serve as markers to monitor the underlying bronchial inflammation. Currently there is no diagnostic marker of bronchial asthma, which, on its own, either confirms or excludes bronchial asthma with appropriate sensitivity and specificity. Clinically the most reliable feature of bronchial asthma that seems to be related closely to the symptomatology still is the presence of eosinophils in peripheral blood, and especially in sputum. Eosinophil-derived products, particularly eosinophil granule proteins, have been investigated as markers of eosinophil participation in the pathogenesis of asthma and, comparable to eosinophil numbers themselves, are possible predictors of impending exacerbations of allergic, as well as intrinsic bronchial asthma. However, clinically their precise value in diagnosing and monitoring of bronchial asthma has not been documented convincingly and requires further investigation. Increasing data suggest that the regulation of eosinophilia is largely conveyed by interleukin-5 (IL-5) released from activated T-helper lymphocytes and possibly other cells. Therefore, T-lymphocyte activation, and especially assessment of systemic and local IL-5 levels, might be of diagnostic value and possibly useful in monitoring of inflammation in bronchial asthma in the future. A possible role and future applications for other markers of inflammation not related to eosinophils in monitoring or diagnosing bronchial asthma need to be established.
Similar content being viewed by others
References
Aalbers, R, de Monchy JGR, Kaufman HF, Smith M, Hoekstra, Vrugt B, Timens W (1993) Dynamics of eosinophil infiltration in the bronchial mucosa before and after the late asthmatic reaction Eur Respir J 6:840–847.
Ackerman SJ, Kephart GM, Habermann TM, Greipp PR, Gleich GJ (1983) Localisation of eosinophil granule major basic protein in human basophils. J Exp Med 158:946–961
Ackerman SJ, Weil GJ, Gleich GJ (1982) Formation of Charcot-Leyden crystals by human basophils. J Exp Med 155:1597–1609
Bachner RL, Johnston RB (1971) Metabolic and bactericidal activities of human eosinophils. Br J Haematol 20:277–285
Baigelman W, Chodosh S, Pizzuto D, Cupples A (1983) Sputum and blood cosinophilia during corticosteroid treatment of acute exacerbations of asthma. Am J Med 75:929–936
Batter MS, Eschenbacher WL, Peters-Golden M (1988) Arachidonic acid metabolism in cultured alveolar macrophages from normal, atopic, and asthmatic subjects. Am Rev Respir Dis 138:1134–1142
Barnes PJ (1989) A new approach to the treatment of asthma. N Engl J Med 321:1517–1527
Beasley R, Roche WR, Roberts JA, Holgate ST (1989) Cellular events in the bronchi in mild asthma and after bronchoprovocation. Am Rev Respir Dis 139:806–817
Bentley AM, Maestrelli P, Saetta M, Fabbri LM, Robinson DS, Bradley BL, Jeffrey PK, Durham SR, Kay AB (1992) Activated T-lymphocytes and eosinophils in the bronchial mucosa in isocyanate-induced asthma. J Allergy Clin Immunol 89:821–829
Bentley AM, Menz G, Storz C, Robinson DS, Bradley B, Jeffrey PK, Durham SR, Kay AB (1992) Identification of T-lymphocytes, macrophages, and activated eosinophils in the bronchial mucosa in intrinsic asthma. Relationship to symptoms and bronchial responsiveness. Am Rev Respir Dis 146:500–506
Blumberg MZ, Buckley JM (1976) The total eosinophil count in asthmatic children. J Allergy Clin Immunol 57:493–498
12. Boner AL, Peroni DG, Piacentini GL, Venge P (1993) Influence of allergen avoidance at high altitude on serum markers of eosinophil activation in children with allergic asthma. Clin Exp Allergy 23 (in press)
Bosso JV, Schwartz LB, Stevenson DD (1991) Tryptase and histamine release during aspirin-induced respiratory reactions. J Allergy Clin Immunol 88:830–837
Bousquet J, Chanez P, Lacoste JY, Barneon G, Ghavanian N, Enander I, Venge P, Ahlstedt S, Simony-Lafontaine J, Godard P, Michel FB (1991) Eosinophilic inflammation in asthma. N Engl J Med 323:1033–1039
Bousquet J, Chanez P, Lacoste JY, Enander I, Venge P, Peterson C, Ahlstedt S, Michel FB, Godard P (1991) Indirect evidence of bronchial inflammation assessed by titration of inflammatory mediators in BAL fluid of patients with asthma. J Allergy Clin Immunol 88:649–660
Bradding P, Feather IH, Howarth PH, Mueller R, Roberts JA, Britten K, Bews JPA, Hunt TC, Okayama Y, Heusser CH, Bullock GR, Church MK, Holgate ST (1992) Interleukin 4 is localized to and released by human mast cells. J Exp Med 176:1381–1386
Bradley BL, Azzawi M, Jacobson M, Assoufi B, Collins JV, Irani AM, Schwartz LB, Durham SR, Jeffrey PK, Kay AB (1991) Eosinophils, T-lymphocytes, mast cells, neutrophils, and macrophages in bronchial biopsy specimens from atopic subjects with asthma: Comparison with biopsy specimens from atopic subjects and relation to bronchial hyperresponsiveness. J Allergy Clin Immunol 88:661–674
Broide DH, Gleich GJ, Cuomo AS, Coburn DA, Federman EC, Schwartz LB, Wasserman SI (1991) Evidence of ongoing mast cell and eosinophil degranulation in symptomatic asthmatic airways. J Allergy Clin Immunol 80:637–648
Broide DH, Lotz M, Cuomo A, Coburn DA, Federman EC, Wasserman SI (1992) Cytokines in symptomatic asthma airways. J Allergy Clin Immunol 89:958–967
Brown HM (1958) Treatment of chronic asthma with prednisolone—Significance of eosinophils in the sputum. Lancet ii:1245–1247
Brown PH, Crompton GK, Greening AP (1991) Proinflammatory cytokines in acute asthma. Lancet 338:590–593
Bruijnzeel PLB, Virchow J-C, Ribs S, Walker C, Verhage J (1993) Lack of increased numbers of low-density eosinophils in the circulation of asthmatic individuals. Clin Exp Allergy 23:261–269
Carlson M, Hakansson L, Peterson C, Stalenheim G, Venge P (1991) Secretion of granule proteins from eosinophils and neutrophils is increased in asthma. J Allergy Clin Immunol 87:27–33
Casale TB, Wood D, Richerson HB, Zehr B, Zavala D, Hunninghake GW (1978) Direct evidence of a role for mast cells in the pathogenesis of antigen-induced bronchoconstriction. J Clin Invest 80:1507–1511
Chanez P, Bousquet J, Couret I, Cornillac L, Barneon, Vic P, Michel FB, Godard P (1991) Increased numbers of hypodense alveolar macrophages in patients with bronchial asthma. Am Rev Respir Dis 144:923–930
Charcot JM, Robin C (1853) Observation de Leucocythemie. C R Mem Soc Biol 5:44–50
Chodosh S (1970) Examination of sputum cells. N Engl J Med 282:854–857
Church MK, Hiroi J (1987) Inhibition of IgE dependent histamine release from human dispersed mast cells by anti-allergic drugs and salbutamol. Br J Pharmacol 90:421–429
Clutterbuck EJ, Hirst EMA, Sanderson CJ (1988) Human Intereukin-5 (IL-5) regulates the production of eosinophils in human bone marrow cultures: Comparison with IL-1, IL-3, IL-6 and GM-CSF. Blood 73:1504–1512
Cookson WOCM, Craddock CF, Benson MK, Durham SR (1989) Falls in peripheral eosinophil counts parallel the late asthmatic response. Am Rev Respir Dis 139:458–462
Corrao WM, Braman SS, Irwin RS (1979) Chronic cough as the sole presenting manifestation of bronchial asthma. N Engl J Med 300:633–637
Corrigan CJ, Haczku A, Gemou-Engesaeth V, Doi S, Kikuchi Y, Takatsu K, Durham SR Kay AB (1993) CD4 T-lymphocytes activation in asthma is accompanied by increased serum concentrations of interleukin-5. Effect of glucocorticoid therapy. Am Rev Respir Dis 147:540–547
Corrigan CJ, Kay AB (1990) CD4 T-lymphocyte activation in acute severe asthma. Am Rev Respir Dis 141:970–977
Crane J, Pearce N, Flatt A, Burgess C, Jackson R, Kwong T, Ball M, Beasley R (1989) Prescribed fenoterol and death from asthma in New Zealand, 1981–83: Case control study. Lancet i:917–922
Crimi E, Chiaramondia M, Milanese M, Rossi GA, Brusasco V (1991) Increased numbers of mast cells in bronchial mucosa after the late-phase asthmatic response to allergen. Am Rev Respir Dis 144:1282–1286
Curschmann H (1883) Ueber Bronchiolitis exudativa und ihr Verhältnis zum asthma nervosum. Dtsch Arch Klin Med 32:1
Dahl R, Venge P, Olsson I (1978) Variations of blood eosinophilia and eosinophil cationic protein in serum in patients with bronchial asthma. Allergy 33:211–215
De Monchy JG, Kaufmann HF, Venge P, Koeter GH, Jansen HM, Sluiter HJ, de Vries K (1985) Bronchoalveolar eosinophilia during allergen-induced late asthmatic reactions. Am Rev Respir Dis 131:373–376
Denburg JA, Dolovich J, Ohtoshi T, Cox G, Gauldie J, Jordana M (1990) The microenvironmental differentiation hypothesis of airway inflammation. Am J Rhinol 4:29–32
Dent LA, Strath M, Mellor AL, Sanderson CJ (1990) Eosinophilia in transgenic mice expressing interleukin-5. J Exp Med 172:1425–1431
Djukanovic R, Wilson JW, Britten KM, Wilson SJ, Walls AF, Roche WR, Howarth PH, Holgate ST (1990) Quantitation of mast cells and eosinophils in the bronchial mucosa of symptomatic atopic asthmatics and healthy control subjects using immunohistochemistry. Am Rev Respir Dis 142:863–871
Dor PJ, Ackerman SJ, Gleich GJ (1984) Charcot-Leyden crystal protein and eosinophil granule major basic protein in sputum of patients with respiratory diseases. Am Rev Respir Dis 130:1072–1077
Durham SR, Kay AB (1985) Eosinophils, bronchial hyperreactivity and late-phase asthmatic reactions. Clin Allergy 15:411–418
Durham SR, Leogering DA, Dunette S, Gleich GJ, Kay AB (1989) Blood eosinophils and eosinophil-derived proteins in allergic asthma. J Allergy Clin Immunol 84:931–936
45. Ehrlich P, (1879) Ueber die speziflschen Granulationen des Blutes. Arch Anat Physiol (Physiol Abt) 571–579
Epstein MRL (1972) Constituents of sputum: A simple method. Ann Intern Med 77:259–265
Ernst P, Habbick B, Suissa S, Hemmelgarn B, Cockroft D, Buist AS, Horwitz RI, McNutt M, Spitzer WO (1993) Is the association between inhaled beta-agonist use and life-threatening asthma because of confounding by severity? Am Rev Respir Dis 148:75–79
Evans PM, O'Conner BJ, Fuller RW, Barnes PJ, Chung KF (1993) Effect of inhaled corticosteroids on peripheral blood eosinophil counts and density profiles in asthma. J Allergy Clin Immunol 91:643–550
Feltelius N, Hallgren R, Venge P (1987) Raised circulating levels of the eosinophil cationic protein in ankylosing spondylitis: Relation with the inflammatory activity and the influence of sulphasalazine treatment. Ann Rheum Dis 46:403–407
Filley WV, Holley KE, Kephart GM, Gleich GJ (1987) Identification by immunofluorescence of eosinophil granule major basic protein in lung tissues of patients with bronchial asthma. Lancet ii:11–16
Frick WE, Sedgwick JB, Busse WW (1989) The appearance of hypodense eosinophils in antigen-dependent late phase asthma. Am Rev Respir Dis 139:1401–1406
Frigas E, Loegering DA, Gleich GJ (1980) Cytotoxic effects of the guinea pig eosinophil major basic protein on tracheal epithelium. Lab Invest 42:35–43
Frigas E, Loegering DA, Solley GO, Farrow GM, Gleich GJ (1981) Elevated levels of the eosinophil granule major basic protein in the sputum of patients with bronchial asthma. Mayo Clin Proc 56:345–353
Fukuda T, Dunette SL, Reed CE, Ackerman SJ, Peters MS, Gleich GJ (1985) Increased numbers of hypodense eosinophils in the blood of patients with bronchial asthma. Am Rev Respir Dis 132:981–985
Gibson PG, Dolovich J, Denburg J, Ramsdale EH, Hargreave FE (1989) Chronic cough: Eosinophilic bronchitis without asthma. Lancet i:1346–1348
Gibson PG, Girgis-Gabardo A, Morris MM, Mattoli S, Kay JM, Dolovich J, Denburg J, Hargreave FE (1989) Cellular characteristics of sputum from patients with asthma and chronic bronchitis. Thorax 44:693–699
Gleich GJ, Adolphson CR (1986) The eosinophil leukocyte: Structure and function. Adv Immunol 39:177–253
Gleich GJ, Loegering DA, Mann KG, Maldonado JE (1976) Comparative properties of the charcot-Leyden crystal protein from human eosinophils. J Clin Invest 57:633–640
Gollasch H (1889) Zur Kenntnis des Asthmatischen Sputums. Fortschr Med 7:361–365
Gordon JR, Post T, Schulman ES, Galli SJ (1991) Characterisation of mouse mast cell TNF-α induction in vitro and in vivo and demonstration that purified human lung mast cells contain TNFα. FASEB J 5:A1009
Gosset P, Tsicopoulos A, Wallaert B, Vannimenus C, Joseph M, Tonnel AB, Capron A (1991) Increased secretion of tumor necrosis factor alpha and interleukin-6 by alveolar macrophages consecutive to the development of the late asthmatic reaction. J Allergy Clin Immunol 88:561–572
Griffin E, Hakansson L, Formgren H, Jörgensen K, Peterson C, Venge P (1991) Blood eosinophil number and activity in relation to lung function in patients with asthma and with eosinophilia. J Allergy Clin Immunol 87:548–557
Gundel RH, Letts LG, Gleich GJ (1991) Human eosinophil major basic protein induces airway constriction and airway hyperresponsiveness in primates. J Clin Invest 87:1470–1473
Hallgren R, Venge P, Wisstedt B (1982) Elevated levels of lactoferrin and eosinophil cationic protein in schizophrenic patients. Br J Psychiatry 140:55–60
Hansel TT, Braunstein JB, Walker C, Blaser K, Bruijnzeel PLB, Virchow J-C, Jr, Virchow C, Sr (1991) Sputum eosinophils from asthmatics express ICAM-1 and HLA-DR. Clin Exp Immunol 86:271–277
Harding SM, Freedman S (1978) A comparison of oral and inhaled steroids in patients with chronic airways obstruction: Features determining response. Thorax 33:214–218
Hargreave FE, Ryan G, Thomson NC, O'Byrne PM, Latimer K, Juniper EF, Dolovich J (1981) Bronchial responsiveness to histamine or methacholine in asthma: Measurement and clinical significance. J Allergy Clin Immunol 68:347–355
Henderson LL, Swedlund HA, VanDellen RG, Marcoux JP, Carryer HM, Peters GA, Gleich GJ (1971) Evaluation of IgE levels in an allergy practice. J Allergy Clin Immunol 48:361–365
Hetzel MR, Clark TJH (1980) Comparison of normal and asthmatic circadian rhythms in peak expiratory flow rate. Thorax 35:732–738
Horn BR, Robin ED, Theodore J, Van Kessel A (1975) Total eosinophil counts in the management of bronchial asthma. N Engl J Med 292:1152–1155
Howarth PH, Durham SR, Lee TH, Kay AB, Church MK, Holgate ST (1985) Influence of albuterol, cromolyn sodium and ipratropium bromide on the airway and circulating mediator response to allergen bronchial provocation in asthma. Am Rev Respir Dis 132:986–992
International Consensus Report on Diagnosis and Treatment of Asthma (1992) National Heart, Lung, and Blood Institute, Publication No. 92–3091. Eur Respir J 5:601–641
Irani AA, Schechter NM, Craig SS, DeBlois G, Schwartz LB (1986) Two types of human mast cells that have distinct neutral protease composition. Proc Natl Acad Sci USA 83:4464–4468
Juliusson S, Holmberg K, Baumgarten CR, Olsson M, Enander I, Pipkorn U (1991) Tryptase in nasal lavage fluid after local allergen challenge. Allergy 46:459–465
Juntunen-Backman K, Järvinen P, Sorva R (1993) Serum eosinophil cationic protein during treatment of asthma in children. J Allergy Clin Immunol 92:34–38
Kauffman HF, van der Belt B, de Monchy JG, Boelens H, Koeter GH, de Vries K (1987) Leukotriene C4 production by normal density and low density eosinophils of atopic individuals and other patients with eosinophilia. J Allergy Clin Immunol 79:611–619
Kay AB, Bacon GD, Mercer BA, Simpson H, Crofton JW (1974) Complement components and IgE in bronchial asthma. Lancet ii:916–920
Kendrick AH, Higgs CMB, Whitfield MJ, Laszlo G (1993) Accuracy of perception of severity of asthma: Patients treated in general practice. Br Med J 307:422–1124
Kita H, Ohnishi T, Okubo Y, Weiler D, Abrams JS, Gleich GJ (1991) Granulocyte/macrophage colony-stimulating factor and interleukin-3 release from human peripheral blood eosinophils and neutrophils. J Exp Med 174:745–748
Klink M, Cline MG, Halonen M, Burrows B (1990) Problems in defining normal limits for serum IgE. J Allergy Clin Immunol 85:440–444
Lacoste J-Y, Bousquet J, Chanez P, Van Vyve T, Simony-Lafontaine J, Lequeu N, Vic P, Enander I, Godard P, Michel FB (1993) Eosinophilic and neutrophilic inflammation in asthma, chronic bronchitis, and chronic obstructive pulmonary disease. J Allergy Clin Immunol 92:537–548
Laitinen LA, Heino M, Laitinen A, Kava T, Haahtela T (1985) Damage of the airway epithelium and bronchial reactivity in patients with asthma. Am Rev Respir Dis 131:599–606
Laitinen LA, Laitinen A, Haahtela T (1992) A comparative study of the effects of an inhaled corticosteroid, budesonide, and a β2-agonist, terbutaline, on airway inflammation in newly diagnosed asthma: A randomized, double blind, parallel-group controlled trial. J Allergy Clin Immunol 90:32–42
Lam S, LeRiche J, Phillips D, Chan-Young M (1987) Cellular and protein changes in bronchial lavage fluid after late asthmatic reaction in patients with red cedar asthma. Am Rev Respir Dis 80:44–50
Lassalle P, Sergant M, Delneste Y, Gosset P, Wallaert B, Zandecki M, Capron A, Joseph M, Tonnel AB (1992) Levels of soluble IL-2 receptor in plasma from asthmatics. Correlations with blood eosinophils, lung function, and corticosteroid therapy. Clin Exp Immunol 87:266–271
Laviolette M, Cormier Y, Loiseau A, Soler P, Leblanc P, Hance AJ (1991) Bronchoalveolar mast cells in normal and farmers and subjects with farmer's lung. Diagnostic, prognostic, and physiologic significance. Am Rev Respir Dis 144:855–860
Leyden E (1872) Zur Kentniss des Bronchial Asthma. Virchows Arch (Pathol Anat) 54:324–344
Lopez AF, Sanderson CJ, Gamble JR, Campell HD, Young IG, Vadas MA (1988) Recombinant human interleukin-5 is a selective activator of human eosinophil function. J Exp Med 167:219–224
Lowell FC (1967) Clinical aspects of eosinophilia in atopic disease. JAMA 202:875–878
Luksza AR, Jones DK (1982) Comparison of whole-blood eosinophil counts in extrinsic asthmatics with acute and chronic asthma. Br Med J 285:1229–1231
Marsh DG, Bias WB, Ishizaka K (1974) Genetic control of basal serum immunoglobulin E levels and its effect on specific reaginic sensitivity. Proc Natl Acad Sci USA 71:3588–3592
Mattoli S, Mattoso VL, Soloperto M, Allegra L, Fasoli A (1991) Cellular and biochemical characteristics of bronchoalveolar lavage fluid in symptomatic nonallergic asthma. J Allergy Clin Immunol 87:794–802
Mattoli S, Soloperto M, Marini M, Fasoli A (1991) Levels of endothelin in the bronchoalveolar lavage fluid of patients with symptomatic asthma and reversible airflow obstruction. J Allergy Clin Immunol 88:376–384
Medici TC, Chodosh S (1972) Sputum cell dynamics in bacterial exacerbations of chronic bronchial disease. Arch Intern Med 129:597–603
Mossman TR, Coffman RL (1987) Two types of mouse helper T cell clones. Implications for immune regulation. Immunol Today. 8:223–227
Motojima S, Frigas E, Loegering DA, Gleich GJ (1989) Toxicity of eosinophil cationic proteins for guinea pig tracheal epithelium in vitro. Am Rev Respir Dis 139:801–805
Moulder J (1980) Bacteriological examination of sputum in cases of acute and chronic bronchitis. In: Orie NGM, Sluiter JH (eds) Bronchitis: An international symposium, April 1960, University of Groningen. Royal Van Gorcum, Assen, pp 27–29
Naylor B (1962) The shedding of the mucosa of the bronchial tree in asthma. Thorax 17:69–72
Olson RL, Little C (1983) Purification and some properties of myeloperoxidase and eosinophil peroxidase from human blood. Biochem J 209:781–787
Olsson I, Venge P, Spitznagel JK, Lehrer RI (1977) Arginine-rich cationic proteins of human eosinophil granules. Comparison of the constituents of eosinophilic and neutrophilic leukocytes. Lab Invest 36:493–500
Owen WF, Rothenberg ME, Silberstein DS, Gasson JC, Stevens RL, Austen KF, Soberman RJ (1987) Regulation of human eosinophil viability, density, and function by granulocyte-macrophage colony-stimulating factor in the presence of T3T fibroblasts. J Exp Med 166: 129–141
Park CS, Lee SM, Uh ST, Kim HT, Chung YT, Kim YH, Choi BW, Hue SH, Lee HB (1993) Soluble interleukin-2 receptor and cellular profiles in bronchoalveolar lavage fluid from patients with bronchial asthma. J Allergy Clin Immunol 91:623–633
Parronchi P, Macchia D, Piccinni M-P, Biswas P, Simonelli C, Maggi E, Ricci M, Ansari AA, Romagnani S (1991) Allergen- and bacterial antigen-specific T-cell clones established from atopic donors show a different profile of cytokine production. Proc Natl Acad Sci USA 88:4538–4542
Peterson CG, Venge P (1983) Purification and characterization of a new cationic protein-eosinophil protein-X (EPX)—from granules of human eosinophils. Immunology 50:19–26
Plant M, Pierce JH, Watson CJ, Hanley-Hyde J, Nordan RP, Paul W (1989) Mast cell lines produce lymphokines in response to cross-linkage of FceRI or to calcium ionophores. Nature 339:64–67
Poulter LW, Rossi GA, Bjermer L, Costabel U, Israel-Biet D, Klech H, Pohl W, Semenzato G (1992) The value of bronchoalveolar lavage in the diagnosis and prognosis of sarcoidosis. Eur Respir Rev 2:75–82
Rackemann FM (1947) A working classification of asthma. Am J Med 3:601–606
Rak S, Löwhagen O, Venge P (1988) The effect of immunotherapy on bronchial hyper-responsiveness and eosinophil cationic protein in pollen-allergic patients. J Allergy Clin Immunol 82:470–480
Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, Corrigan C, Durham SR, Kay AB (1992) Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med 326:298–304
Rothenberg ME, Owen WF, Silberstein DS, Soberman RJ, Austen KF, Stevens RL (1988) Human eosinophils have prolonged survival, enhanced functional properties, and become hypodense when exposed to human interleukin-3. J Clin Invest 81:1986–1992
Saetta M, Di Stefano A, Maestrelli P, Ferraresso A, Drigo R, Potena A, Ciaccia A, Fabbri LM (1993) Activated T-lymphocytes and macrophages in bronchial mucosa of subjects with chronic bronchitis. Am Rev Respir Dis 147:301–306
Sanerkin NG, Evans DMD (1965) The sputum in bronchial asthma: Pathognomonic patterns. J Pathol Bacteriol 89:535–541
Schüler M, Costabel U, Virchow J-C, Jr, Kortsik C, Hasse J, Matthys H, Kroegel C (1993) Serum eosinophil cationic protein (ECP) levels in disease. A prospective study on 549 patients. J Allergy Clin Immunol 91:A179
Sears MR, Burrows B, Flannery EM, Herbison GP, Hewitt CJ, Holdaway MD (1991) Relation between airway responsiveness and serum IgE in children with asthma and in apparently normal children. N Engl J Med 325:1067–1071
Sears MR, Taylor DR, Print CG, Lake DC, Li Q, Flannery EM, Yates DM, Lucas MK, Herbison GP (1990) Regular inhaled beta-agonist treatment in bronchial asthma. Lancet 336: 1391–1396
Sladek K, Szczeklik A (1993) Cysteinyl leukotrienes overproduction and mast cell activation in aspirin-provoked bronchospasm in asthma. Eur Respir J 6:391–399
Slifman NR, Venge P, Peterson CG, McKean DJ, Gleich GJ (1989) Human eosinophil-derived neurotoxin and eosinophil protein X are likely the same protein. J Immunol 143:2317–2322
Spitzer WO, Suissa S, Ernst P, Horwitz RI, Habbick B, Cockroft D, Boivin J-F, McNutt M, Buist AS, Rebuck AS (1992) The use of beta-agonists and the risk of death and near death from asthma. N Engl J Med 326:501–506
Tai PC, Spry CJ, Peterson C, Venge P, Olsson I (1984) Monoclonal antibodies distinguish between storage and secreted forms of eosinophil cationic protein. Nature 309:182–184
Taylor KJ, Luksza AR (1987) Peripheral blood eosinophil counts and bronchial responsiveness. Thorax 42:452–456
Tomioka M, Ida S, Ishihara T, Takashima T (1984) Mast cells in bronchoalveolar lumen of patients with bronchial asthma. Am Rev Respir Dis 129:1000–1005
Tunon de Lara JM, Okayama Y, McEuen AR, Heusser CH, Church MK, Walls AF (1993) Mast cell proteases can degrade interleukin-4 (IL-4). Eur Respir J 6:367s
Vanhoutte PM (1988) Epithelium-derived relaxing factor(s) and bronchial hyperreactivity. Am Rev Respir Dis 138:S24-S30
Venge P (1990) The human eosinophil in inflammation. Agents Actions 29:122–126
Venge P, Dahl R, Fredens K, Peterson CGB (1988) Epithelial injury by human eosinophils. Am Rev Respir Dis 138:S54-S57
Venge P, Henrikson J, Dahl R (1991) Eosinophils in exercise-induced asthma. J Allergy Clin Immunol 88:699–704
Venge P, Roxin LE, Olsson I(1977) Radioimmunoassay of human eosinophil cationic protein. Br J Haematol 37:331–335
Viera VG, Prolla J-C (1979) Clinical evaluation of eosinophils in the sputum. J Clin Pathol 32:1054–1057
Virchow C, Sr (1973) Intrinsic asthma. Prax Klin Pneumol 27:578–591
Virchow C, Sr, Möller E, Debelic M (1971) IgE-Serumspiegelbestimmungen bei chronischobstruktiven Atemwegsleiden. Pneumologie-Pneumology 145:428–440
Virchow J-C, Jr, Hölscher U, Virchow C, Sr (1992) Sputum ECP levels correlate with parameters of airflow obstruction. Am Rev Respir Dis 146:604–606
Virchow J-C, Jr, Kroegel C, Hage U, Kortsik C, Matthys H, Werner P (1993) Comparison of sputum ECP levels in bronchial asthma and chronic bronchitis. Allergy 48(Suppl):112–118
Virchow J-C, Jr, Walker C, Häfner D, Kortsik C, Werner P, Engelstätter R, Blaser K, Luttmann W, Matthys H, Kroegel C (1993) Cytokine networking in asthmatic inflammation. Its role in lymphocyte and eosinophil activation. Eur Respir J 6 (Suppl 17):514s
Walker C, Bode E, Boer L, Hansel TT, Blaser K, Virchow J-C, Jr (1992) Allergic and nonallergic asthmatics have distinct patterns of T-cell activation and cytokine production in peripheral blood and bronchoalveolar lavage. Am Rev Respir Dis 146:109–115
Walker C, Kaegi MK, Braun P, Blaser K (1991) Activated T cells and eosinophilia in bronchoalveolar lavages from subjects with asthma correlated with disease severity. J Allergy Clin Immunol 88:935–942
Walker C, Virchow J-C, Jr, Bruijnzeel PLB, Blaser K (1991) T cell subsets and their soluble products regulate eosinophilia in allergic and nonallergic asthma. J Immunol 146:1829–1835
Wardlaw AJ, Dunette S, Gleich GJ, Collins JV, Kay AB (1988) Eosinophils and mast cells in bronchoalveolar lavage in subjects with mild asthma. Am Rev Respir Dis 137:62–69
Weller PF, Goetzl EJ, Austen KF (1980) Identification of human eosinophil lysophospholipase as the constituent of Charcot-Leyden crystals. Proc Natl Acad Sci USA 77:7440–7443
Wempe JB, Tammeling EP, Koeter GH, Hakansson L, Venge P, Postma DS (1992) Blood eosinophil numbers and activity during 24 hours: Effects of treatment with budesonide and bambuterol. J Allergy Clin Immunol 90:757–765
Wenzel SE, Fowler AA III, Schwartz LB (1988) Activation of pulmonary mast cells by bronchoalveolar allergen challenge: In vivo release of histamine and tryptase in atopic subjects with and without asthma. Am Rev Respir Dis 137:1002–1008
Wittig HJ, Belloit J, De Fillippi I, Royal G (1980) Age-related serum immunoglobulin E levels in healthy subjects and in patients with allergic disease. J Allergy Clin Immunol 66:305–313
Wodnar-Filipowicz A, Heusser CH, Moroni C (1989) Production of the haemopoietic growth factors GM-CSF and interleukin-3 by mast cells in response to IgE receptor-mediated activation. Nature 339:150–152
Wong BJ, Dolovich J, Ramsdale EH, O'Byrne P, Gontovnick L, Denburg JA, Hargreave FE (1992) Formoterol compared with beclomethasone and placebo on allergen-induced asthmatic responses. Am Rev Respir Dis 146:1156–1160
Wong DTW, Weller PF, Galli SJ, Elovic A, Rand TH, Gallagher GT, Chiang T, Chou MY, Matossian K, McBride J, Todd R (1990) Human eosinophils express transforming growth factor α. J Exp Med 172:673–681
Wuethrich B, Schindler C, Zemp E, Zellweger J-P, SAPALDIA-Team (1993) Prevalence of pollinosis in the adult population of Switzerland (SAPALDIA-study). Allergy 48(Suppl):10
Yunginger JW, Gleich GJ (1973) Seasonal changes in serum and nasal IgE concentrations. J Allergy Clin Immunol 51:174–186
Zimmerman B, Lanner A, Enander I, Zimmerman RS, Peterson CGB, Ahlstedt S (1993) Total blood eosinophils, serum eosinophil cationic protein and eosinophil protein X in childhood asthma: Relation to disease status and therapy. Clin Exp Allergy 23:564–570
Author information
Authors and Affiliations
Additional information
Offprint requests to: J.-C. Virchow, Jr.
Rights and permissions
About this article
Cite this article
Virchow, JC., Kroegel, C., Walker, C. et al. Cellular and immunological markers of allergic and intrinsic bronchial asthma. Lung 172, 313–334 (1994). https://doi.org/10.1007/BF00172846
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00172846