Summary
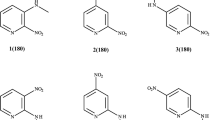
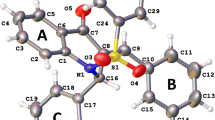
The side-chain conformations of psychoactive phenothiazine drugs in crystals are different from those of biologically inactive ring sulfoxide metabolites. This study examines the potential energies, molecular conformations and electrostatic potentials in chlorpromazine, levomepromazine (methotrimeprazine), their sulfoxide metabolites and methoxypromazine. The purpose of the study was to examine the significance of the different crystal conformations of active and inactive phenothiazine derivatives, and to determine why phenothiazine drugs lose most of their biological activity by sulfoxidation. Quantum mechanics and molecular mechanics calculations demonstrated that conformations with the side chain folded over the ring structure had lowest potential energy in vacuo, both in the drugs and in the sulfoxide metabolites. In the sulfoxides, side chain conformations corresponding to the crystal structure of chlorpromazine sulfoxide were characterized by stronger negative electrostatic potentials around the ring system than in the parent drugs. This may weaken the electrostatic interaction of sulfoxide metabolites with negatively charged domains in dopamine receptors, and cause the sulfoxides to be virtually inactive in dopamine receptor binding and related pharmacological tests.
Similar content being viewed by others
References
Jørgensen, A., In Bridges, J.W. and Chasseaud, L.F. (Eds.), Progress in Drug Metabolism, Vol. 9, Taylor and Francis, London, 1986, pp. 111–174.
Dahl, S.G., Therap. Drug Monit., 4 (1982) 33.
Axelsson, R. and Mårtensson, E., Curr. Therap. Res., 21 (1977) 561.
Aravagiri, M., Hawes, E.M. and Midha, K.K., J. Pharm. Sci., 73 (1984) 1383.
Marder, S.M., Hubbard, J.W., VanPutten, T. and Midha, K.K., Psychopharmacology, 98 (1989) 433.
Dahl, S.G., Strandjord, R.E. and Sigfusson, S., Europ. J. Clin. Pharmacol., 11 (1977) 305.
Gottschalk, L.A., Dinovo, E., Biener, R. and Nandi, B.R., J. Pharm. Sci., 67 (1978) 155.
Axelsson, R. and Mårtensson, E., Curr. Therap. Res., 28 (1980) 463.
Dahl, S.G. and Strandjord, R.E., Clin. Pharmacol. Therap., 21 (1977) 437.
Dahl, S.G. (Review), In Usdin, E., Dahl, S.G., Gram, L.F. and Lingjærde, O. (Eds.), Clinical Pharmacology in Psychiatry. Neuroleptic and Antidepressant Research, Macmillan, London, 1981, pp. 125–137.
Creese, I., Manian, A.A., Prosser, T.D. and Snyder, S.H., Eur. J. Pharmacol. 47 (1978) 291.
Dahl, S.G. and Hall, H., Psychoparmacology 74 (1981) 101.
Morel, E., Lloyd, K.G. and Dahl, S.G., Psychopharmacology, 92 (1987) 68.
Dahl, S.G. and Refsum, H., Eur. J. Pharmacol., 37 (1976) 241.
Dahl, S.G., Hjorth, M. and Hough, E., Mol. Pharmacol., 20 (1982) 409.
Hough, E., Hjorth, M. and Dahl, S.G., Acta Crystallogr., B38 (1982) 2424.
Hough, E., Hjorth, M. and Dahl, S.G., Acta Crystallogr., C41 (1985) 383.
Hough, E., Wold, E. and Dahl, S.G., Acta Crystallogr., C41 (1985) 386.
Dahl, S.G., Hough, E. and Hals, P.-A., Biochem. Pharmacol. 35 (1986) 1263.
Marsau, P. and Gauthier, J., Acta Crystallogr. C29 (1973) 992.
Viterbo, D., Hansen, L.K., Hough, E. and Dahl, S.G., Acta Crystallogr., C42 (1986) 889.
McDowell, J.J.H., Acta Crystallogr., B25 (1969) 2175.
Singh, U.C., Weiner, P.K., Caldwell, J.W. and Kollman, P.A., Assisted model building with energy refinement. AMBER UCSF Version 3.0, Dept. Pharmaceutical Chemistry, University of California, San Francisco, CA 94143, 1986.
Weiner, S.J., Kollman, P.A., Nguyen, D.T. and Case, D.A., J. Comput. Chem. 7 (1986) 230.
Jorgensen, W.L., Chandrasekhar, J., Madura, J.D., Impey, R.W. and Klein, M.L., J. Chem. Phys., 79 (1983) 926.
Singh, U.C. and Kollman, P.A., J. Comp. Chem., 5 (1984) 129.
Binkley, J.S., Whiteside, R.A., Krishnan, R., Seeger, R., Defrees, D.J., Schlegel, H.B., Topiol, S., Kahn, L.R. and Pople, J.A., GAUSSIAN 80, Quantum Chemistry Program Exchange, 1980.
Blackmore, W.R. and Abrahams, S.C., Acta Crystallogr., 8 (1955) 329.
Pierce, L. and Hayashi, M., J. Chem. Phys., 35 (1961) 479.
Abrahams, S.C., Acta Crystallogr., 10 (1957) 417.
Feder, W., Dreizler, H., Rudolph, D.H. and Typke, V., Z. Naturforsch., 24a (1969) 266.
Ferrin, T.E., Huang, C.C., Jarvis, L.E. and Langridge, R., J. Mol. Graphics, 6 (1988) 2.
Ferrin, T.E., Huang, C.C., Jarvis, L.E. and Langridge, R., J. Mol. Graphics, 6 (1988) 13.
Richards, F.M., Annu. Rev. Biophys. Bioeng., 6 (1977) 151.
Connolly, M.L., Science, 221 (1983) 709.
Strange, P.G., T.I.N.S. 13 (1990) 373.
Dahl, S.G., Edvardsen, Ø. and Sylte, I., Proc. Natl. Acad. Sci. U.S.A., 88 (1991) 8111.
Neve, K.A., Tester, B.A., Henningsen, R.A., Spanoyannis, A. and Neve, R.L., Mol. Pharmacol. 39 (1991) 733.
Zichi, D.A. and Rossky, P.J., J. Chem. Phys., 84 (1986) 1712.
Miller, R.J., Horn, A.S. and Iversen, L.L., Mol. Pharmacol., 10 (1974) 759–766.
Sylte, I. and Dahl, S.G., J. Pharm. Sci., 80 (1991) 735.
Sylte, I. and Dahl, S.G., Pharm. Res., 8 (1991) 462.
Burgen, A.S.V., Roberts, G.C.K. and Feeney, J., Nature 253, (1975) 753.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dahl, S.G., Kollman, P.A., Rao, S.N. et al. Structural changes by sulfoxidation of phenothiazine drugs. J Computer-Aided Mol Des 6, 207–222 (1992). https://doi.org/10.1007/BF00123377
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00123377