Abstract
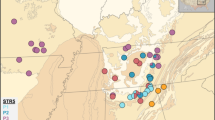
Genetic variability and divergence at 21 enzyme loci were studied in and between Italian populations of the cave spiders Nesticus eremita (13 populations), N. menozzii and N. sbordonii (one population each). The three species differ with respect to the degree of specialization to cave life, dispersion ability, isolation of populations, abundancy, extent of the distribution area, and range from the troglophilic and widespread N. eremita to the troglobitic N. sbordonii, endemic to a single cave in the Central Appennines.
Heterozygosity ranges from 0.05 to 0.15 in N. eremita populations and appears to be largely controlled by the occurrence and the extent of gene flow among populations. The relatively low polymorphism levels of N. menozzii (H=0.081) and N. sbordonii (H=0.106) are also associated with reduced gene flow and small population sizes.
Genetic distances between N. eremita populations vary considerably and are strictly related to the geographical distances involved, again indicating a major role of gene flow in determining the patterns of genetic differentiation between populations. This view is strongly supported by the results of a principal component analysis applied to the gene-frequency data. Estimates of genetic divergence between species suggest that the major cladogenetic events leading to complete separation of these three Nesticus species occurred in the Middle-late Pliocene.
Similar content being viewed by others
References
Avise, J. C., 1976. Genetic differentiation during speciation; p. 106–122. In: F. J., Ayala (ed), Molecular evolution. Sinauer, Sunderland, Mass.
Ayala, F. J., 1975. Genetic differentiation during the speciation process. Evolut. Biol. 8: 1–75.
Ayala, F. J., Powell, J. R., Tracey, M. L., Mourão, C. A. & Pérez-Salas, S., 1972. Enzyme variability in the Drosophila willistoni group. IV. Genic variation in natural populations of Drosophila willistoni. Genetics 70: 113–139.
Bullini, L. & Sbordoni, V., 1980. Electrophoretic studies on gene-enzyme systems: microevolutionary processes and phylogenetic inference. Boll. Zool. 47 (suppl.): 95–112.
Brewer, G. J., 1970. An introduction to isozyme techniques. Academic Press, New York.
Brignoli, P. M., 1972. Catalogo dei ragni cavernicoli italiani. Quad. Speleologia Circolo Speleologico Romano 1: 5–211.
Brignoli, P. M., 1979. Ragni d'Italia: 31. Specie Cavernicole nuove o interessanti. Quad. Mus. Speleologia V. Rivera 10: 3–48.
Chakraborty, R. & Nei, M., 1977. Bottleneck effects on average heterozygosity and genetic distance with the stepwise mutation model. Evolution 31: 347–356.
Dickerson, R. E., 1974. The structure of cytochrome c and the rates of molecular evolution. J. molec. Evol. 3: 161–178.
Kimura, M. & Crow, J. F., 1964. The number of alleles that can be maintained in a finite population. Genetics 49: 725–738.
Langley, C. H. & Fitch, W. M., 1974. An examination of the constancy of the rate of molecular evolution. J. mol. Evol. 3: 161–177.
Levene, H., 1949. On a matching problem arising in genetics. A. math. Statist. 20: 91–94.
Levins, R., 1968. Evolution in changing environments. Princeton Univ. Press, Princeton, New Jersey.
Nei, M., 1972. Genetic distance between populations. Am. Nat. 106: 283–292.
Nei, M., 1975. Molecular population genetics and evolution. North-Holland Publ. Comp. Amsterdam. Oxford.
Nei, M., Maruyama, T. & Chakraborty, R., 1975. The bottleneck effect and genetic variability in populations. Evolution 29: 1–10.
Orlóci, L., 1975. Multivariate analysis in vegetation research. Junk, The Hague.
Ruffo, S., 1955. Le attuali conoscenze della fauna cavernicola della regione Pugliese. Memorie Biogeogr. adriat. 3: 1–143.
Sarich, V. M., 1977. Rates, sample size and the neutrality hypothesis for electrophoresis in evolutionary studies. Nature 265: 24–28.
Sbordoni, V., 1980. Strategie adattative negli animali cavernicoli: uno studio di genetica ed ecologia di popolazione. Accad. naz. Lincei. Contrti Centro Linceo interdisciplinare Sci. matemat. Applic. 51: 61–100.
Sbordoni, V., Allegrucci, G., Caccone, A., Cesaroni, D., Cobolli Sbordoni, M. & De, Matthaeis, E., 1980. A preliminary report of the genetic variability in troglobitic Bathysciinæ: Leptodirus hohenwarti and two Orostygia species (Coleoptera, Catopidae). Fragm. ent. 15: 327–336.
Sbordoni, V., Allegrucci, G., Caccone, A., Cesaroni, D., Cobolli Sbordoni, M. & De, Matthaeis, E., 1981. Genetic variability and divergence in cave populations of Troglophilus cavicola and T. andreinii (Orthoptera, Rhaphidophoridae). Evolution 35: 226–233.
Sbordoni, V., Caccone, A., De, Matthaeis, E. & Cobolli Sbordoni, M., 1980b. Biochemical divergence between cavernicolous and marine Sphaeromidae and the Mediterranean salinity crisis. Experientia 36: 48–49.
Sbordoni, V., Cobolli Sbordoni, M. & De, Matthaeis, E., 1979. Divergenza genetica tra popolazioni e specie ipogee ed epigee di Niphargus (Crustacea, Amphipoda). Lav. Soc. Ital. Biogeogr. (n.s.) 6: 329–351.
Schmitt, L. H., 1978. Genetic variation in isolated populations of the Australian bushrat. Rattus fuscipes. Evolution 32: 1–14.
Selander, R. K. & Kaufman, D. W., 1973. Self-fertilization and genetic population structure in a colonizing land snail. Proc. natn. Acad. Sci. U.S.A. 70: 1186–1190.
Sneath, P. H. A. & Sokal, R. R., 1973. Numerical taxonomy. The principles and practice of numerical classification. Freeman, San Francisco.
Taylor, C. E. & Powell, J. R., 1977. Microgeographic differentiation of chromosomal and enzyme polymorphisms in D. persimilis. Genetics 85: 681–695.
Valentine, J. W. & Ayala, F. J., 1978. Adaptive strategies in the sea; p. 323–345. In: B., Battaglia and J. A., Beardmore (eds), Marine organisms. Genetics, ecology and evolution. NATO Conferences series (4), 2. Plenum Press, New York.
Zimmerman, E. G., Kilpatrick, C. W. & Hart, B. J., 1978. The genetics of speciation in the rodent genus Peromyscus. Evolution 32: 565–579.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cesaroni, D., Allegrucci, G., Caccone, A. et al. Genetic variability and divergence between populations and species of Nesticus cave spiders. Genetica 56, 81–92 (1981). https://doi.org/10.1007/BF00055410
Issue Date:
DOI: https://doi.org/10.1007/BF00055410