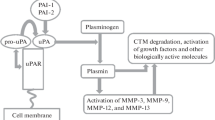
In order to invade and spread cancer cells must degrade extracellular matrix proteins. This degradation is catalysed by the concerted action of several enzymes, including the serine protease plasmin. Several experimental studies have shown that inhibition of plasmin formation reduces cancer cell invasion and metastasis, indicating a critical role of this proteolytic pathway in these processes. In order to further study the role of plasmin in cancer progression, we have characterized urokinase-type plasminogen activator (uPA) mediated plasmin formation in three human breast cancer cell lines. Using monoclonal antibodies against uPA and its receptor uPAR, we have investigated the contribution of uPA and uPAR to invasive capacity in an in vitro invasion assay. MDA-MB-231 BAG cells were found to express high protein levels of uPA, uPAR and PAI-1. MDA-MB-435 BAG cells produced low amounts of uPA, PAW and moderate amounts of uPAR, whereas MCF-7 BAG cells showed low levels of uPA, uPAR and PAM protein. In a plasmin generation assay MDA-MB-231 BAG cells were highly active in mediating plasmin formation, which could be abolished by adding either an anticatalytic monoclonal antibody to uPA (clone 5) or an anti-uPAR monoclonal antibody (clone R3), which blocks binding of uPA to uPAR. The two other cell lines lacked the capacity to mediate plasmin formation. In the Matrigel invasion assay the cells showed activity in this order: MCF-7 BAG < MDA-MB-435 BAG < MDA-MB-231 BAG. Testing MDA-MB-231 BAG cells in the Matrigel invasion assay revealed that invasion could be inhibited in a dose-dependent manner either by the clone 5 uPA antibody or by the clone R3 uPAR antibody, suggesting that the cell surface uPA system is actively involved in this invasive process. It is concluded that these three cell lines constitute a valuable model system for in vitro studies of the role of cell surface uPA in cancer cell invasion and has application in the search for novel compounds which inhibit mechanisms involved in uPA-mediated plasmin generation on cancer cells.
Similar content being viewed by others
References
Hollas W, Blasi F and Boyd D, 1991, Role of the urokinase receptor in facilitating extracellular matrix invasion by cultured colon cancer. Cancer Res, 51, 3690–5.
Ossowski L, 1988, In vivo invasion of modified chorioallantoic membrane by tumor cells: The role of cell surface-bound urokinase. J Cell Biol, 107, 2437–45.
Quax PHA, Pedersen N, Masucci MT, et al. 1991, Complementation between urokinase-producing and receptor-producing cells in extracellular matrix degradation. Cell Regulation, 2, 793–803.
Ossowski L, Clunie G, Masucci MT and Blasi F, 1991, In vivo paracrine interaction between urokinase and its receptor: Effect on tumor invasion. J Cell Biol, 115, 1107–12.
Albini A, Melchiori A, Santi L, Liotta LA, Brown PD and Stetler-Stevenson WG, 1991, Tumor cell invasion inhibited by TIMP-2. J Natl Cancer Inst, 83, 775–9.
Ossowski L and Reich E, 1983, Antibodies to plasminogen activator inhibit human tumor metastasis. Cell, 35, 611–19.
Ossowski L, Russo-Payne H and Wilson EL, 1991, Inhibition of urokinase-type plasminogen activator by antibodies: The effect on dissemination of a human tumor in the nude mouse. Cancer Res, 51, 274–81.
Crowley CW, Cohen RL, Lucas BK, Liu G, Shuman MA and Levinson AD, 1993, Prevention of metastasis by inhibition of the urokinase receptor. Proc Natl Acad Sci USA, 90, 5021–25.
Kobayashi H, Gotoh J, Fujie M, Shinohara H, Moniwa N and Terao T, 1994, Inhibition of metastasis of Lewis lung carcinoma by a synthetic peptide within the growth factor-like domain of urokinase in the experimental and spontaneous metastasis model. Int J Cancer, 57, 727–33.
Kook YH, Adamski J, Zelent A and Ossowski L, 1994, The effect of antisense inhibition of urokinase receptor in human squamous cell carcinoma on malignancy. EMBO J, 17, 3983–91.
Schultz RM, Silberman S, Persky B, Bajkowski AS and Carmichael DF, 1988, Inhibition by human recombinant tissue inhibitor of metalloproteinases of human amnion invasion and lung colonization by murine B16-F10 melanoma cells. Cancer Res, 48, 5539–5545.
DeClerck YA, Perez N, Shimada H, Boone TC, Langley KE and Taylor SM, 1992, Inhibition of metastasis in cells transfected with an inhibitor of metalloproteinases. Cancer Res, 52, 701–8.
Prendiville J, Crowther D, Thatcher N, et al. 1993, A phase I study of intraveneous bryostatin-1 in patients with advanced cancer. Br J Cancer, 68, 418–24.
Danø K, Andreasen PA, Grøndahl-Hansen J, Kristensen P, Nielsen LS and Skriver L, 1985, Plasminogen activators, tissue degradation, and cancer. Adv Cancer Res, 44, 139–266.
Danø K, Nielsen LS, Pyke C and Kellerman GM, 1988, Plasminogen activators and neoplasia. In: Kluft C, ed. Tissue-type plasminogen activator (t-PA): Physiological and clinical aspects. Boca Raton: CRC Press, pp. 19–46.
Roldan AL, Cubellis MV, Masucci MT, et al. 1990, Cloning and expression of the receptor for human urokinase plasminogen activator, a central molecule in cell surface, plasmin dependent proteolysis. EMBO J, 9, 467–74.
Andreasen PA, Georg B, Lund LIZ, Riccio A and Stacey SN, 1990, Plasminogen activator inhibitors: Hormonally regulated serpins. Molec Cell Endocr, 68, 1–19.
Ellis V, Behrendt N and Danó K, 1991, Plasminogen activation by receptor-bound urokinase: A kinetic study with both cell-associated and isolated receptor. J Biol Chem, 266, 12752–8.
Ellis V, Scully MF and Kakkar VV, 1989, Plasminogen activation initiated by single-chain urokinase-type plasminogen activator. Potentiation by U937 monocytes. J Biol Chem, 264, 2185–8.
Ellis V, Wun TC, Behrendt N, Renne E and Danø K, 1990, Inhibition of receptor-bound urokinase by plasminogen activator inhibitors. J Biol Chem, 265, 9904–8.
Cubellis MV, Wun TC and Blasi F, 1990, Receptor-mediated internalization and degradation of urokinase is caused by its specific inhibitor PAI-1. EMBO J, 9, 1079–85.
Estreicher A, Mühlhauser J, Carpentier J-L, Orci L and Vassali JD, 1990, The receptor for urokinase type plasminogen activator polarizes expression of the protease to the leading edge of migrating monocytes and promotes degradation of enzyme inhibitor complexes. J Cell Biol, 111, 783–92.
Olson D, Pöllänen J, Heyer-Hansen G, et al. 1992, Internalization of the urokinase-plasminogen activator inhibitor type-1 complex is mediated by the urokinase receptor. J Biol Chem, 267, 9129–9133.
Nykjær A, Petersen CM, Møller B, et al. 1992, Purified alpha 2-macroglobulin receptor/LDL receptor-related protein binds urokinase plasminogen activator inhibitor type-1 complex. Evidence that the alpha 2-macroglobulin receptor mediates cellular degradation of urokinase receptor-bound complexes. J Biol Chem, 267, 14543–6.
Murphy G, Ward R, Gavrilovic J and Atkinson S, 1992, Physiological mechanisms for metalloproteinase activation. In: Birkedal-Hansen H, Werb Z, Welgus H, Van Wart H, eds. Matrix metalloproteinases and inhibitors. Gustav Fisher Verlag: Stuttgart, pp. 245–55.
Lyons RM, Gentry LE, Purchio AF and Moses HL, 1990, Mechanism of activation of latent recombinant transforming growth factor 1 by plasmin. J Cell Biol, 110, 1361–7.
Sato Y and Rifkin DB, 1989, Inhibition of endothelial cell movement by pericytes and smooth muscle cells: activation of a latent transforming growth factor-β1-like molecule by plasmin during co-culture. J Cell Biol, 109, 309–15.
Campbell PG, Novak JF, Yanosick TB and McMaster JH, 1992, Involvement of plasmin system in dissociation of the insulin-like growth factor-binding protein complex. Endocrinology, 130, 1401–12.
Saksela O and Rifkin DB, 1990, Release of basic fibroblast growth factor-heparin sulfate complexes from endothelial cells by plasminogen activator-mediated proteolytic activity. J Cell Biol, 110, 767–75.
Brünner N, Thompson EW, Spang-Thomsen M, Rygaard J, Danø K and Zwiebel JA, 1992, LacZ transduced human breast cancer xenografts as an in vivo model for the study of invasion and metastasis. Eur J Cancer, 28A, 1989–95.
Price J, Turner D and Cepko C, 1987, Lineage analysis in the vertebrate system by retrovirus-mediated gene transfer. Proc Natl Acad Sci USA, 84, 156–60.
Mosmann T, 1983, Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Meth, 65, 55–63.
Camiolo SM, Suita MR and Madeja JM, 1982, Improved medium for extraction of plasminogen activator from tissue. Prep Biochem, 12, 297–305.
Ronne E, Heyer-Hansen G, Brünner N, et al. 1995, Urokinase receptor in breast cancer tissue extracts. Enzyme-linked immunosorbent assay with a combination of mono- and polyclonal antibodies. Breast Cancer Res Treat, 33, 199–207.
Ronne E, Behrendt N, Ploug M, et al. 1994, Quantitation of the receptor for urokinase plasminogen activator by enzyme-linked immunosorbent assay. J Immunol Meth, 167, 91–101.
Rosenquist C, Thorpe SM, Danø K and Grøndahl-Hansen J, 1993, Enzyme-linked immunosorbent assay of urokinase-type plasminogen activator (uPA) in cytosolic extracts of human breast cancer tissue. Breast Cancer Res Treat, 28, 223–30.
Grendahl-Hansen J, Christensen IJ, Rosenquist C, et al. 1993, High levels of urokinase-type plasminogen activator and its inhibitor PAM in cytosolic extracts of breast carcinomas are associated with poor prognosis. Cancer Res, 53, 2513–21.
Andreasen PA, Nielsen LS, Kristensen P, Grøndahl-Hansen J, Skriver L and Dana K, 1986, Plasminogen activator inhibitor from human fibrosarcoma cells binds urokinase-type plasminogen activator, but not its proenzyme. J Biol Chem, 261, 7644–51.
Nielsen LS, GrØndahl-Hansen J, Andreasen PA, Skriver L, Zeuthen J and Danø K, 1986, Enzyme-linked immunosorbent assay for human urokinase-type plasminogen activator and its proenzyme using a combination of monoclonal and polyclonal antibodies. J Immunoassay, 7, 209–28.
Behrendt N, Rønne E, Ploug M, et al. 1990, The human receptor for urokinase plasminogen activator. NH2-terminal amino acid and glycosylation variants. J Biol Chem, 265, 6453–60.
Ellis V, Behrendt N and Danø K, 1993, Cellular receptor for urokinase-type plasminogen activator: Function in cell-surface proteolysis. Meth Enzym, 223, 223–33.
Ronne E, Behrendt N, Ellis V, Ploug M, Danø K and Høyer-Hansen G, 1991, Cell-induced potentiation of the plasminogen activation system is abolished by a monoclonal antibody that recognizes the NH2-terminal domain of the urokinase receptor. FEBS Lett, 288, 233–6.
Repesh LA, 1989, A new in vitro assay for quantitating tumor cell invasion. Invasion and Metastasis, 9, 192–208.
Nielsen LS, Andreasen PA, Grøndahl-Hansen J, Huang JY, Kristensen P and Danø K, 1986, Monoclonal antibodies to human 54,000 molecular weight plasminogen activator inhibitor from fibrosarcoma cells — inhibitor neutralization and one-step affinity purification. Thrombosis and Haemostasis, 55, 206–12.
Keijer J, Linders M, van Zonneveld AJ, Ehrlich HJ, de Boer JP and Pannekoek H, 1991, The interaction of plasminogen activator inhibitor 1 with plasminogen activators (tissue-type and urokinase-type) and fibrin: Localization of interaction sites and physiologic relevance. Blood, 78, 401–9.
Solberg H, Romer J, Brünner N, et al. 1994, A cleaved form of the receptor for urokinase-type plasminogen activator in invasive transplanted human and murine tumors. Int J Cancer, 58, 877–81.
Rose DP, Connolly JM and Liu XH, 1994, Effects of linoleic acid on the growth and metastasis of two human breast cancer cell lines in nude mice and the invasive capacity of these cell lines in vitro. Cancer Res, 54, 6557–62.
Busso N, Belin D, Failly-Crépin C and Vassalli JD, 1987, Glucocorticoid modulation of plasminogen activators and of one of their inhibitors in the human mammary carcinoma cell line MDA-MB-231. Cancer Res, 15, 364–70.
Romer J, Pyke C, Lund LR, et al. 1994, Expression of uPA and its receptor by both neoplastic and stromal cells during xenograft invasion. Int J Cancer, 57, 553–60.
Azzam HS, Arand G, Lippman ME and Thompson EW, 1993, Association of MMP-2 activation potential with metastatic progression in human breast cancer cell lines independent of MMP-2 production. J Nail Cancer Inst. 85, 1758–64.
Pyke C, Ralfkiær E, Huhtala P, Hurskainen T, Danø K and Tryggvason K, 1992, Localization of messenger RNA for Mr 72,000 and 92,000 type IV collagenase in human skin cancers by in situ hybridization. Cancer Res, 52, 1336–41.
Pyke C, Graem N, Ralfkiær E, et al. 1993, Receptor for urokinase is present in tumor-associated macrophages in ductal breast carcinoma. Cancer Res, 53, 1911–15.
Thompson EW, Paik S, Brünner N, et al. 1992, Association of increased basement membrane invasiveness with absence of estrogen receptor and expression of vimentin in human breast cancer cell lines. J Cell Phys, 150, 534–44.
Pourreau-Schneider N, Delori P, Boutière B, et al. 1989, Modulation of plasminogen activator systems by matrix components in two breast cancer cell lines: MCF-7 and MDA-MB-231. J Natl Cancer Inst, 81, 259–66.
Danø K, Behrendt N, Brünner N, Ellis V, Ploug M and Pyke C, 1994, The urokinase receptor. Protein structure and role in plasminogen activation and cancer invasion. Fibrinolysis, 8, 189–203.
Pedersen H, Brünner N, Francis D, et al. 1994, Prognostic impact of urokinase, urokinase receptor, and type 1 plasminogen activator inhibitor in squamous and large cell lung cancer tissue. Cancer Res, 54, 4671–5.
Ganesh S, Sier CFM, Heerding MM, Griffioen G, Lamers CB and Verspaget HW, 1994, Urokinase receptor and colorectal cancer survival. The Lancet, 344, 401–2.
Grøndahl-Hansen J, Peters HA, van Putten WLJ, et al. Prognostic significance of the receptor for urokinase plasminogen activator in breast cancer. Clin Cancer Res, in press.
Høyer-Hansen G, Ronne E, Solberg H, et al. 1992, Urokinase plasminogen activator cleaves its cell surface receptor releasing the ligand-binding domain. J Biol Chem, 267, 18224–9.
Ploug M, Eriksen J, Plesner T, Hansen NE and Danø K, 1992, A soluble form of the glycolipid-anchored receptor for urokinase-type plasminogen activator is secreted from peripheral blood leukocytes from patients with paroxysmal nocturnal hemoglobinuria. Eur J Biochem, 208, 397–404.
Pedersen N, Schmitt M, Ronne E, et al. 1993,A ligand-free, soluble urokinase receptor is present in the ascitic fluid from patients with ovarian cancer. J Clin Invest, 92, 2160–7.
Duggan C, Maguire T, McDermott E, O'Higgins H, Fennelly JJ and Duffy MJ, 1995, Urokinase plasminogen activator and urokinase plasminogen activator receptor in breast cancer. Int J Cancer, 61, 597–600.
Nekarda N, Schmitt M, Ulm K, et al. 1994, Prognostic impact of urokinase-type plasminogen activator and its inhibitor PAI-1 in completely resected gastric cancer. Cancer Res, 54, 2900–7.
Kuhn W, Pache L, Schmalfeldt B, et al. 1994, Urokinase (uPA) and PAI-1 predict survival in advanced ovarian cancer patients (FIGO III) after radical surgery and platinum-based chemotherapy. Gynecol Oncol, 55, 401–9.
Pyke C, Kristensen P, Ralfkiær E, Eriksen J and Danø K, 1991, The plasminogen activation system in human colon cancer: Messenger RNA for the inhibitor PAI-1 is located in endothelial cells in the tumor stroma. Cancer Res, 51, 4067–71.
Pappot H, Gârdsvoll H, Romer J, et al. 1995, Plasminogen activator inhibitor type 1 in cancer: Therapeutic and prognostic implications. Biol Chem Hoppe-Seyler, 376, 259–67.
Liu G, Shuman MA and Cohen RL, 1995, Co-expression of urokinase, urokinase receptor and PAI-I is necessary for optimum invasiveness of cultured lung cancer cells. Int J Cancer, 60, 501–6.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Holst-Hansen, C., Johannessen, B., Hoyer-Hansen, G. et al. Urokinase-type plasminogen activation in three human breast cancer cell lines correlates with their in vitro invasiveness. Clin Exp Metast 14, 297–307 (1996). https://doi.org/10.1007/BF00053903
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00053903