Abstract
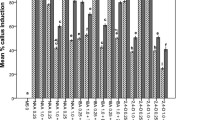
Adventitious shoot regeneration was compared among leaf, stem and petal explants of carnation (Dianthus caryophyllus L.) cv. Scania on MS medium containing different concentrations of 6-benzyladenine (BA) and α-naphthaleneacetic acid (NAA). High frequency regeneration was obtained only from petal explants on the media containing 5 to 10 μM BA with or without 5 μM NAA. Among the cytokinins tested, N-2-chloro-4-pyridyl-N′-phenylurea and N-1,2,3-thiadiazol-5-yl-N′-N′-phenylurea were more effective than BA, kinetin, N6-2-isopentenyl adenine and zeatin on regeneration from petal explants. Although, high frequency shoot regeneration was obtained from all petal explants harvested from various developmental stages of buds, a significant decrease in regeneration capacity was observed in the explants obtained from fully-opened flowers. High frequency shoot regeneration was also obtained from the petal explants of cvs. Coral. Lena, Nora and White Sim, and an interspecific cultivar Eolo using the method developed in this study.
Similar content being viewed by others
Abbreviations
- NAA:
-
α-naphthaleneacetic acid
- BA:
-
6-benzyladenine
- GA3 :
-
gibberellic acid
- 2iP:
-
N6-2-isopentenyl adenine
- KT-30:
-
N-2-chloro-4-pyridyl-N′-phenylurea (also called 4PU)
- TDZ:
-
N-1,2,3-thiadiazol-5-yl-N′-phenylurea (also called thidiazuron)
References
Chi G-L, Barfield DG, Sim G-E & Pua E-C (1990) Effect of AgNO3 and aminoethoxyvinylglycine on in vitro shoot and root organogenesis from seedling explants of recalcitrant Brassica genotypes. Plant Cell Rep. 9: 195–198
Chraibi BKM, Latche A, Roustan J-P & Fallot J (1991) Stimulation of shoot regeneration from cotyledons of Helianthus annuus by the ethylene inhibitors, silver and cobalt. Plant Cell Rep. 10: 204–207
Gasser CS & Fraley RT (1989) Genetically engineering plants for crop improvement. Science 244: 1293–1299
Gimelli F, Ginatta G, Venturo R, Positano S & Buiatti M (1984) Plantlet regeneration from petals and floral induction in vitro in the mediterranean carnation (Dianthus caryophyllus L.). Riv.Ortoflorofrutt.It. 68: 107–121
Kakehi M (1979) Studies on the tissue culture of carnation. V. Induction of redifferentiated plants from petal tissue. Bull. Hiroshima Agr. Coll. 6; 159–166 (in Japanese)
Leshem B (1986) Carnation plantlets from vitrified plants as a source of somaclonal variation. HortScience 21: 320–321
Lu C-Y, Nugent G, Wardley-Richardson T, Chandler SF, Young R & Dalling MJ (1991) Agrobacterium-mediated transformation of carnation (Dianthus caryophyllus). Bio/Technology 9: 864–868
Matsuta N & Hirabayashi T (1989) Embryogenic cell lines from somatic embryos of grape (Vitis vinifera L.). Plant Cell Rep. 7: 684–687
Miller RM, Kaul V, Hutchinson JF & Richards D (1991) Adventitious shoot regeneration in carnation (Dianthus caryophyllus) from axillary bud explants. Ann. Bot. 67: 35–42
Mok MC, Mok DWS, Armstrong DJ, Shudo K, Isogai Y & Okamoto T (1982) Cytokinin activity of N-phenyl-N′-1,2,3-thiadiazol-5-ylurea (thidiazuron). Phytochemistry 21: 1509–1511
Murashige T & Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15: 473–497
Nakano M & Mii M (1992) Protoplast culture and plant regeneration of several species and cultivars in the genus Dianthus. Plant Cell Rep. 11: 225–228
Nugent G, Wardley-Richardson T & Lu C-Y (1991) Plant regeneration from stem and petal of carnation (Dianthus caryophyllus L.). Plant Cell Rep. 10: 477–480
Petru E & Landa Z (1974) Organogenesis in isolated carnation plant callus tissue cultivated in vitro. Biol. Plant. 16: 450–453
Purnhauser L, Medgyesy P, Czako M, Dix PJ & Marton L (1987) Stimulation of shoot regeneration in Triticum aestivum and Nicotiana plumbaginifolia Viv. tissue cultures using the ethylene inhibitor AgNO3. Plant Cell Rep. 6: 1–4
Suezawa K, Matsuta N, Omura M & Yamaki S (1988) Plantlet regeneration from cell suspensions of kiwifruit (Actinidia chinensis Planch. var. chinensis). Scientia Hort. 37: 123–128
Takahashi S, Shudo K, Okamoto K, Yamada K & Isogai Y (1978) Cytokinin activity of N-phenyl-N′-(4-pyridyl)urea derivatives. Phytochemistry 17: 1201–1207
van der Krol AR, Lenting PE, Veenstra J, van der Meer IM, Koes RE, Gerats AGM, Mol JNM & Stuitje AR (1988) An antisense chalcone synthase gene in transgenic plants inhibits flower pigmentation. Nature 333: 866–869
Weryszko E & Hempel M (1979) Studies on in vitro multiplantlets of carnation. II. The histological analysis of multiplantlets formation. Acta Hort. 91: 323–331
Woodson WR (1987) Changes in protein and mRNA populations during the senescence of carnation petals. Physiol. Plant. 71: 495–502
Woodson WR (1991) Biotechnology of floricultural crops. HortScience 26: 1029–1033
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nakano, M., Hoshino, Y. & Mii, M. Adventitious shoot regeneration from cultured petal explants of carnation. Plant Cell Tiss Organ Cult 36, 15–19 (1994). https://doi.org/10.1007/BF00048310
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00048310