Abstract
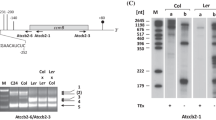
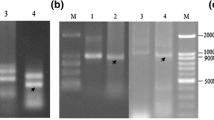
RNA editing and NH2-terminal processing of subunit 6 (atp6) of the mitochondrial Fo-ATPase complex has been investigated for the normal (fertile) and Ogura (male-sterile) radish cytoplasms to determine if previously identified differences between the Ogura atp6 locus and its normal radish counterpart are associated with cytoplasmic male sterility. Analysis of cDNA clones from five different sterile and fertile radish lines identified one C-to-U transition, which results in the replacement of a proline with a serine, in several of the lines. No editing of atp6 transcripts was observed in two lines, Scarlet Knight (normal radish) and sterile CrGC15 (Ogura radish). This is the first example of a naturally occurring plant mitochondrial gene that is not edited. The Ogura atp6 polypeptide is synthesized with a predicted NH2-terminal extension of 174 amino acids in contrast to the nine amino acid extension found in normal radish. In spite of the lack of similarity between the two extensions, NH2-terminal sequence analysis indicates that both polypeptides are processed to yield identical core proteins with a serine as the NH2-terminal residue. These results indicate that ATPase subunit 6 is synthesized normally in Ogura radish, and that it is unlikely that the atp6 locus is associated with Ogura cytoplasmic male sterility.
Similar content being viewed by others
References
Begu D, Graves P-V, Domec C, Arselin G, Litvak S, Araya A: RNA editing of wheat mitochondrial ATP synthase subunit 9: direct protein and cDNA sequencing. Plant Cell 2: 1283–1290 (1990).
Bland MM, Levings CSIII, Matzinger DF: The ATPase subunit 6 gene of tobacco mitochondria contains an unusual sequence. Curr Genet 12: 475–481 (1987).
Bonen L, Bird S: Sequence analysis of the wheat mitochondrial atp6 gene reveals a fused upstream reading frame and markedly divergent N termini among plant ATP6 proteins. Gene 73: 47–56 (1988).
Bonhomme S, Budar F, Lancelin D, Small I, Defrance MC, Pelletier G: Sequence and transcript analysis of the NcoI 2.5 Ogura-specific fragment correlated with cytoplasmic male sterility in Brassica cybrids. Mol Gen Genet 235: 340–348 (1992).
Bonnard G, Gualberto JM, Lamattina L, Grienenberger JM: RNA editing in plant mitochondria. Crit Rev Plant Sci 10: 503–524 (1992).
Braun CJ, Brown GG, Levings CSIII: Cytoplasmic male sterility. In: Herrmann RG (ed) Cell Organelles pp. 219–239. Spinger-Verlag, New York (1992).
Chapdelaine Y, Bonen L: The wheat mitochondrial gene for subunit-I of the NADH dehydrogenase complex — a trans-splicing model for this gene-in-pieces. Cell 65: 465–472 (1991).
Covello PS, Gray MW: Differences in editing at homologous sites in messenger RNAs from angiosperm mitochondria. Nucl Acids Res 18: 5189–5196 (1990).
D'Aquila RT, Bechtel LJ, Videler JA, Eron JJ, Gorczyca P, Kaplen JC: Maximizing sensitivity and specificity of PCR by preamplification heating. Nucl Acids Res 19: 3749–3755 (1991).
Dewey RE, Levings CSIII, Timothy DH: Nucleotide sequence of ATPase subunit 6 gene of maize mitochondria. Plant Physiol 79: 914–919 (1985).
Dewey RE, Levings CSIII, Timothy DH: Novel recombinations in the maize mitchondrial genome produce a unique transcriptional unit in the Texas male-sterile cytoplasm. Cell 44: 439–449 (1986).
Edwardson JR: Cytoplasmic male sterility. Bot Rev 36: 341–420 (1979).
Glaubitz JC, Carlson JE: RNA editing in the mitochondria of a conifer. Curr Genet 22: 163–165 (1992).
Grabau E, Havlik M, Gestland R: Chimeric organization of two genes for the soybean mitochondrial ATPase subunit 6. Curr Genet 13: 83–89 (1988).
Handa H, Nakajima K: RNA editing of atp6 transcripts from male-sterile and normal cytoplasms of rapeseed (Brassica napus L.) FEBS Lett 310: 111–114 (1992).
Hanson MR, Conde MF: Function and variation of cytoplasmic genomes: lessons from cytoplasmic-nuclear interactions affecting male fertility in plants. Int Rev Cytol 94: 213–267 (1985).
Hanson MR, Folkerts O: Structure and function of the higher plant mitochondrial genome. Int Rev Cytol 141: 129–165 (1992).
Harlow E, Lane D: Antibodies: A Laboratory Manual, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY (1988).
Kadowaki K-I, Suziki T, Kazama S: A chimeric gene containing the 5′ portion of atp6 is associated with cytoplasmic male sterility of rice. Mol Gen Genet 224: 10–16 (1990).
Kaul MLH: Male Sterility in Higher Plants. Springer-Verlag, New York (1988).
Kawasaki ES: Amplification of RNA. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds), PCR Protocals: A guide to methods and applications, pp. 21–29. Academic Press, San Diego (1990).
Kempken F, Mullen JA, Pring DR, Tang HV: RNA editing of sorghum mitochondrial atp6 transcripts changes 15 amino acids and generates a carboxy-terminus identical to yeast. Curr Genet 20: 417–422 (1991).
Kolodner R, Tewari KK: Physicochemical characterication of mitochondrial DNA from pea leaves. Proc Natl Acad Sci USA 69: 1930–1934 (1972).
Krishnasamy S, Makaroff CA: Characterization of the radish mitochondrial orfB locus: possible relationship with male sterility in Ogura radish. Curr Genet 24: 256–163 (1993).
Kumar R, Levings CSIII: RNA editing of a chimeric maize mitochondrial gene transcript is sequence specific. Curr Genet 23: 154–159 (1993).
Laemmli EK: Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685 (1970).
Leaver CJ, Hack E, Forde BJ: Protein synthesis by isolated plant mitochondria. Meth Enzymol 43: 476–484 (1983).
Levings CSIII: The Texas cytoplasm of maize: cytoplasmic male sterility and disease susceptibility. Science 250: 942–947 (1990).
Lu BW, Hanson MR: A single nuclear gene specifies the abundance and extent of RNA editing of a plant mitochondrial transcript. Nucl Acids Res 20: 5699–5703 (1992).
Macfarlane JL, Wahleithner JA, Wolstenholme DR: A broad bean mitochondrial atp6 gene with an unusually simple, non-conserved 5′ region. Curr Genet 18: 87–91 (1990).
Makaroff CA, Apel IJ, Palmer JD: The atp6 coding region has been disrupted and a novel reading frame generated in the mitochondrial genome of cytoplasmic male-sterile radish. J Biol Chem 264: 11706–11713 (1989).
Makaroff CA, Apel IJ, Palmer JD: Characterization of radish mitochondrial atpA — influence of nuclear background on transcription of atpA-associated sequences and relationship with male sterility. Plant Mol Biol 15: 735–746 (1990).
Makaroff CA, Apel IJ, Palmer JD: The role of coxI-associated repeated sequences in plant mitochondrial DNA rearrangements and radish cytoplasmic male sterility. Curr Genet 19: 183–190 (1991).
Makaroff CA, Palmer JD: Mitochondrial DNA rearrangements and transcriptional alterations in the male-sterile cytoplasm of Ogura radish. Mol Cell Biol 8: 1474–1480 (1988).
Mancino G, Tzagoloff A: Assembly of the mitochondrial membrane system: Sequence analysis of a yeast mitochondrial ATPase gene containing the oli-2 and oli-4 loci. Cell 20: 507–517 (1980).
Michon, Galante M, Velours J: NH2-terminal requence of the isolated yeast ATP synthase subunit 6 reveals post-translational cleavage. Eur J Biochem 172: 621–625 (1988).
Mullen JA, Pring DR, Kempken F, Ferguson J, Chase CD: Sorghum mitochondrial atp6 — divergent amino extensions to a conserved core polypeptide. Plant Mol Biol 20: 71–79 (1992).
Nivison HT, Hanson MR: Identification of a mitochondrial protein associated with cytoplasmic male sterility in Petunia. Plant Cell 1: 1121–1130 (1989).
Nowak C, Kuck U: RNA editing of the mitochondrial atp9 transcript from wheat. Nucl Acids Res 18: 7164 (1990).
Ogura H: Studies on the new male-sterility in Japanese radish, with special references to the utilization of this sterility towards the practical raising of hybrid seeds. Mem Fac Agric Kagoshima Univ 6: 39–78 (1968).
Salazar RA, Pring DR, Kempken F: Editing of mitochondrial atp9 transcripts from two sorghum lines. Curr Genet 20: 483–486 (1991).
Sanger F, Nicklen S, Coulson AR: DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74: 5463–5467 (1977).
Schuster W, Brennicke A: Pseudocopies of the ATPase a-subunit gene in Oenothera mitochondria are present on different circular molecules. Mol Gen Genet 204: 29–35 (1986).
Schuster W, Brennicke A: RNA editing of ATPase subunit 9 in Oenothera mitochondria. FEBS Lett 268: 252–256 (1990).
Schuster W, Brennicke A: RNA editing in ATPase subunit-6 messenger RNAs in Oenothera mitochondria-A new termination codon shortens the reading frame by 35 amino acids. FEBS Lett 295: 97–101 (1991).
Singh M, Brown GG: Suppression of cytoplasmic male sterility by nuclear genes alters expression of a novel mitochondrial gene region. Plant Cell 3: 1349–1362 (1991).
Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC: Measurement of protein using bicinchoninic acid. Anal Biochem 150: 76–85 (1985).
Speicher DW: Microsequencing with PVDF membranes: efficient electroblotting, direct protein absorption and sequencer modification In: Hugli TE (ed) Techniques in Protein Chemistry, pp. 24–35. Academic Press, San Diego (1988).
Stern DB, Newton KL: Isolation of plant mitochondrial RNA. Meth Enzymol 118: 488–496 (1986).
Tempst P, Riviere L: Examination of automated polypeptide sequencing using standard phenyl isothiocyanate reagent and subpicomole high-performance liquid chromatographic analysis. Anal Biochem 183: 290–300 (1989).
Ward GC, Levings CSIII: The protein-encoding gene T-urf13 is not edited in maize mitochondria. Plant Mol Biol 17: 1083–1088 (1991).
Wintz H, Hanson MR: A termination codon is created by RNA editing in the Petunia mitochondrial atp9 gene transcript. Curr Genet 19: 61–64 (1991).
Wissinger B, Brennicke A, Schuster W: Regenerating good sense — RNA editing and transsplicing in plant mitochondria. Trends Genet 8: 322–328 (1992).
Wissinger B, Schuster W, Brennicke A: Trans splicing in Oenothera mitochondria — nadl-messenger RNAs are edited in exon and trans-splicing group-II intron sequences. Cell 65: 473–482 (1991).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Krishnasamy, S., Grant, R.A. & Makaroff, C.A. Subunit 6 of the Fo-ATP synthase complex from cytoplasmic male-sterile radish: RNA editing and NH2-terminal protein sequencing. Plant Mol Biol 24, 129–141 (1994). https://doi.org/10.1007/BF00040580
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00040580