Abstract
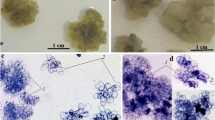
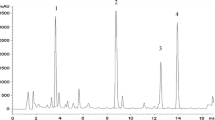
Callus and suspension cultures were established from Quassia amara, a member of the Simaroubaceae. Analysis of the tissue culture showed that quassin was present in both callus and suspension cultures. The effect of variation in auxin and cytokinins on both callus growth and the presence of quassin was examined. The suspension culture was grown in a 7 liter bioreactor when good yields of quassin were achieved.
Similar content being viewed by others
Abbreviations
- IAA:
-
indole-3-acetic acid
- IBA:
-
indolebutyric acid
- 2,4-D:
-
2,4-dichlorophenoxyacetic acid
- NAA:
-
naphthaleneacetic acid
- 6BA:
-
6-benzyladenine
- IpA:
-
N6 (Δ-isopentenyl) adenine
- IpAR:
-
N6 (Δ isopentenyl) adenine riboside
- td:
-
doubling time
References
Allan EJ, Scragg AH & Pugh K (1988) Cell suspension culture of Picrasma quassioides: the development of a rapidly growing, shear resistant cell line capable of quassin formation. J. Plant Physiol. 132: 176–183
Anderson LA, Harris A & Phillipson JD (1983) Production of cytotoxic canthin-6-one alkaloids by Ailanthus altissima plant cell cultures. J. Nat. Prod. 46: 374–378
Anderson LA, Hay CA, Roberts MF & Phillipson JD (1986a) Studies on Ailanthus altissima cell suspension culture. Plant Cell Reports 5: 387–390
Anderson LA, Phillipson JD & Roberts MF (1986b) Alkaloid production by plant cells. In: Webb C & Mavituna F (Eds) Animal Cells, Process Possibilities (pp 172–192). Ellis Horwood, Chichester
Chan KL, O'Neill MJ, Phillipson JD & Warhurst D (1986) Plants as sources of antimalarial drugs. Part 3. Eurycoma longifolia. Planta Med. 52: 105–107
Dixon RA (1985) Isolation and maintenance of callus and cell suspension cultures. In: Dixon RA (Ed) Plant Cell Culture: A Practical Approach (pp 1–20). IRL Press, Oxford
Furuya T, Kojima H & Syonok H (1971) Regulation of nicotine biosynthesis by auxins in tobacco callus tissues. Phytochem. 10: 1529–1532
Gamborg OL, Miller RA & Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Expl. Cell Res. 50: 151–158
Jaziri M, Homes J & Vanhaeien M (1987) Production of quassinoids by tissue culture of Ailanthus altissima. Phytochem. 26: 999–1000
Odjo A, Piart J, Polonsky J & Roth M (1981) Etude de l'effect insecticide de deux quassainoids sur des larves de Locusta migratoria magratoroides. C.R. Acad. Sci. Paris, Ser III, 293: 241–244
Pierre A, Robert-Gero M, Tempete C & Polonsky J (1980) Structural requirements of quassinoids for the inhibition of cell transformation, Biochem. Biophys. Res. Commun. 93: 67686
Polonsky J (1973) Quassinoid bitter principles. Fortsch. Chem. Org. Natur. 30: 101–150
Robins RJ & Rhodes MJC (1984) High-performance liquid chromatographic methods for the analysis and purification of quassinoids from Quassia amara. J. Chromatogr. 283: 436–441
Scragg AH & Allan EJ (1986) Production of the triterpenoid quassin in callus and cell suspension cultures of Picrasma quassioides Bennett. Plant Cell Reports 5: 356–359
Scragg AH, Morris P, Allan EJ, Bond P & Fowler MW (1987) Effect of scale-up on serpentine formation by Catharanthus roseus suspension cultures. Enzyme Microb. Technol. 9: 619–624
Stafford A, Smith L & Fowler MW (1983) Regulation of product synthesis in cell culture of Catharanthus roseus. I. Growth related indole alkaloid accumulation in batch culture. Plant Cell Tiss. Org. Cult. 4: 83–94
Trager W, Polonsky J (1981) Antimalarial activity of quassinoids against chloroquinone-resistant Plasmodium faliciparum in vitro. Am. J. Trop. Med. Hyg. 30: 531–537
Tri Mvan, Polonsky J. Merienne O & Severet T (1981) Soularubinone, a new antileukemic quassinoid from Soulamea tomentosa. J. Nat. Prod. 44: 279–284
Zenk MH, El-Shagi H & Shulte U (1975) Anthraquinone production by cell suspension cultures of Morinda citrifolia. Planta Med. Suppl. 70: 101–135
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Scragg, A.H., Ashton, S., Steward, R.D. et al. Growth of and quassin accumulation by cultures of Quassia amara . Plant Cell Tiss Organ Cult 23, 165–169 (1990). https://doi.org/10.1007/BF00034427
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00034427