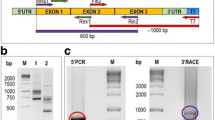
Abstract
One gene and two cDNAs encoding three different β-tubulins (TUB1, TUB2, TUB3) of pea have been cloned and sequenced. The derived amino acid sequences show between 92% and 96% identity relative to one another and to most other β-tubulins of higher plants and green algae. Two notable extremes are the high similarity of 98% between pea TUB3 and maize β-tubulin 2 and the relatively low similarity (90%) of the hypocotyl-specific β-tubulin 1 of soybean to the pea sequences. These similarities do not reflect the molecular phylogeny but rather differences in evolutionary rate of β-tubulins which are differentially regulated during plant development. Genomic Southern blots reveal a β-tubulin gene family in pea with at least four separate members including two TUB1 genes, one TUB2 gene and one TUB3 gene. This contradicts an earlier report by Rahaet al. (Plant Mol Biol 9: 565–571, 1987) suggesting a tandem repeat organization of tubulin genes in pea. The pea TUB1 gene has two introns in identical positions compared to the β-tubulin genes fromArabidopsis and soybean. In an attempt to reconstruct the universal ancestor of all present-day tubulin genes the intron positions in 38 different α- and β-tubulin genes from plants, animals, fungi and protozoa were compared. This comparison shows that the primordial gene probably had many introns (more than 20) separating ‘protoexons’ of 15 to 20 codons in agreement with the ‘exon theory of genes’. It also supports the view that, during the course of evolutions introns have shifted and were deleted preferentially in the 3′ part of the genes. Similar observations have been made previously for other genes. They can be interpreted in terms of a homologous recombination of genes with their modified (incorrectly spliced) and reverse-transcribed pre-mRNAs.
Similar content being viewed by others
References
Brinkmann H, Cerff R, Salomon M, Soll J: Cloning and sequence analysis of cDNAs encoding the cytosolic precursors of subunits GapA and GapB of chloroplast glyceraldehyde-3-phosphate dehydrogenases from pea and spinach. Plant Mol Biol 13: 81–94 (1989).
Cech TR: The generality of self-splicing RNA: relationship to nuclear RNA splicing. Cell 44: 207–210 (1986).
Cerff R, Hundrieser J, Friedrich R: Subunit B of chloroplast glyceraldehyde-3-phosphate dehydrogenase is related to β-tubulin. Mol Gen Genet 204: 44–51 (1986).
Cleveland DW, Sullivan KF: Molecular biology and genetics of tubulin. Annu Rev Biochem 54: 331–365 (1985).
Dibb NJ, Newman AJ: Evidence that introns arose at proto-splice sites. EMBO J 8: 2015–2021 (1989).
Doolittle F: The origin and function of intervening sequences in DNA: a review. Am Nat 130: 915–928 (1987).
Dorit RL, Schoenbach L, Gilbert W: How big is the universe of exons. Science 250: 1377–1382 (1990).
Federoff N: Comparison of host strains for cloning maize DNA in bacteriophage Lambda. Plant Mol Biol Rep 1: 27–29 (1983).
Feinberg AP, Vogelstein B: A technique for radiolabelling DNA restriction endonuclease fragments to high specific activity. Anal Biochem 137: 266 (1984).
Fosket DE: Cytoskeletal proteins and their genes in higher plants. In: Stumpf P, Conn E (eds) The Biochemistry of Plants, Vol. 15. Molecular Biology, pp. 392–454, Academic Press, New York (1989).
Frischauf A-M, Lehrbach H, Poustka A, Murray N: Lambda replacement vectors carrying polylinker sequences. J Mol Biol 170: 827–842 (1983).
Gilbert W: The exon theory of genes. Cold Spring Harbor Symp Quant Biol 52: 901–905 (1987).
Gilbert W, Marchionni M, McKnight G: On the antiquity of introns. Cell 46: 151–154 (1986).
Guiltinan MJ, Ma D-P, Barker RF, Bustos MM, Cyr RJ, Yadegari R, Fosket DE: The isolation, characterization and sequence of two divergent β-tubulin genes from soybean. Plant Mol Biol 10: 171–184 (1987).
Han I-S, Jongewaard I, Fosket DE: Limited expression of a diverged β-tubulin gene during soybean (Glycine max [L.] Merr.) development. Plant Mol Biol 16: 225–234 (1991).
Harper JF, Mages W: Organization and structure ofVolvox β-tubulin genes. Mol Gen Genet 213: 315–324 (1988).
Hussey PJ, Haas N, Hunsperger J, Larkin J, Snustad DP, Silflow CD: The β-tubulin gene family inZea mays: two differentially expressed β-tubulin genes. Plant Mol Biol 15: 957–972 (1990).
Hussey PJ, Lloyd CW, Gull K: Differential and developmental expression of β-tubulins in a higher plant. J Biol Chem 263: 5474–5479 (1988).
Lambowitz AM: Infectious introns. Cell 56: 323–326 (1989).
Lee MGS, Lewis SA, Wilde CD, Cowan NJ: Evolutionary history of a multigene: an expressed human β-tubulin gene and three processed pseudogenes. Cell 33: 477–487 (1983).
Liaud M-F, Zhang DX, Cerff R: Diffential intron loss and endosymbiotic transfer of chloroplast glyceraldehyde-3-phosphate genes to the nucleus. Proc Natl Acad Sci USA 87: 8918–8922 (1990).
Maniatis T, Fritsch EF, Sambrook G: Construction of genomic libraries. In: Molecular cloning: A Laboratory Manual, pp. 270–307. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY (1982).
Marchionni M, Gilbert W: The triosephosphate isomerase gene from maize: Introns antedate the plant-animal divergence. Cell 46: 133–141 (1986).
Marks MD, West J, Weeks DP: The relatively large beta-tubulin family ofArabidopsis contains a member with an unusual transcribed\5′ noncoding sequence. Plant Mol Biol 10: 91–104 (1987).
Martinez P, Martin WF, Cerff R: Structure, evolution and anaerobic regulation of a nuclear gene encoding cytosolic glyceraldehyde-3-phosphate dehydrogenase from maize. J Mol Biol 208: 551–565 (1989).
Neff NN, Thomas JH, Grisafi P, Botstein D: Isolation of the β-tubulin gene from yeast and demonstration of its essential functionin vivo. Cell 33: 211–219 (1983).
Quigley F, Brinkmann H, Martin WF, Cerff R: Strong functional GC pressure in a light regulated maize gene encoding subunit GAPA of chloroplast glyceraldehyde-3-phosphate dehydrogenase: implications for the evolution of GAPA pseudogenes. J Mol Evol 29: 412–421 (1989).
Quigley F, Martin WF, Cerff R: Intron conservation across the prokaryote-eukaryote boundary: Structure of the nuclear gene for chloroplast glyceraldehyde-3-phosphate dehydrogenase from maize. Proc Natl Acad Sci USA 85: 2672–2676 (1988).
Raha D, Sen K, Biswas BB: cDNA cloning of β-tubulin gene and organization of tubulin genes inVigna radiata (mung bean) genome. Plant Mol Biol 9: 565–571 (1987).
Sato N: Nucleotide sequence of a pseudogene for pea phytochrome reminiscent of an incorrect splicing event. Nucl Acids Res 18: 3632 (1990).
Schwarz-Sommer Zs, Gierl A, Klösgen RB, Wienand U, Peterson PA, Saedler H: The Spm (En) transposable element controls the excision of a 2kb DNA insert at thewx-m8 allele ofZea mays. EMBO J 3: 1021–1028 (1984).
Senapathy P: Origin of eukaryotic introns: A hypothesis, based on codon distribution statistics in genes, and its implications. Proc Natl Acad Sci USA 83: 2133–2137 (1986).
Senapathy P: Possible evolution of splice-junction signals in eukaryotic genes from stop codons. Proc Natl Acad Sci USA 85: 1129–1133 (1988).
Sharp PA: On the origin of RNA splicing and introns. Cell 42: 397–400 (1985).
Silflow CD, Oppenheimer DG, Kopczak SD, Ploense SE, Ludwig SR, Haas N, Snustad P: Plant tubulin genes: structure and differential expression during development. Devel Genet 8: 435–460 (1987).
Sullivan KF, Cleveland DW: Identification of conserved isotype-defining variable region sequences for four vertebrate β-tubulin polypeptide classes. Proc Natl Acad Sci USA 83: 4327–4331 (1986).
Sullivan KF, Lau ITY, Cleveland DW: Apparent gene conversion between β-tubulin genes yields multiple regulatory pathways for a single β-tubulin polypeptide isotype. Moll Cell Biol 5: 2454–2465 (1985).
Wilson AC, Carlson SS, White TJ: Biochemical evolution. Annu Rev Biochem 46: 573–639 (1977).
Woodson SA, Cech TR: Reverse self-splicing of theTetrahymena group I intron: implications for the directionality of splicing and for intron transposition. Cell 57: 335–345 (1989).
Wu CI, Li WH, Shen JJ, Scarpulla RC, Limbach KJ, Wu R: Evolution of cytochrome c genes and pseudogenes. J Mol Evol 23: 61–75 (1986).
Youngblom J, Schloss JA, Silflow CD: The two β-tubulin genes ofChlamydomonas reinhardtii code for identical proteins. Mol Cell Biol 4: 2686–2696 (1984).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Liaud, MF., Brinkmann, H. & Cerff, R. The β-tubulin gene family of pea: Primary structures, genomic organization and intron-dependent evolution of genes. Plant Mol Biol 18, 639–651 (1992). https://doi.org/10.1007/BF00020007
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00020007