Summary
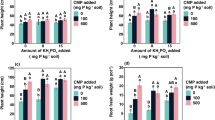
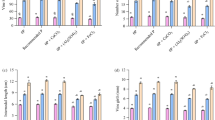
The effect of CaCO3 and iron on the availability of iron, manganese phosphorus and calcium was studied in the greenhouse on pea (Pisum sativum L.) crop on a light textured soil, which was marginal in exchangeable calcium. Addition of calcium carbonate caused significant increase in dry matter yield with no added iron at both the stages of crop growth. But yeild intended to decrease with 8% CaCO3 at 75 days of crop growth. Dry matter yield also increased with the addition of iron upto 10 ppm at 45 days and upto 5 ppm at 75 days. The iron concentration and uptake decreased with the increase in CaCO3 and increased with the application of iron at both the stages of crop growth. The application of iron and CaCO3 decreased concentration and uptake of phosphorus significantly at both the stages.
Like phosphorus, concentration and uptake of manganese also decreased with the increase in added CaCO3 upto 8% and iron upto 20 ppm at 45 and 75 days. The concentration of calcium increased with the addition of CaCO3 to the extent of 50 and 40% with 8% CaCO3 at 45 and 75 days, while the uptake of calcium increased more than 3 folds at 45 days and more than 2 folds at 75 days. The concentration of calcium decreased with the application of iron upto 20 ppm but the uptake at 45 days increased upto 10 ppm and at 75 days upto 5 ppm and then decreased.
The concentration of Fe, P and Ca decreased at 75 days and that of Mn increased while the uptake of all these nutrients increased at 2nd stage due to higher dry matter.
Similar content being viewed by others
References
Bolle Jones E. W., The effect of varied nutrient levels on the concentration and distribution of manganese within the potato plants. Plant and Soil 6, 45–60 (1955).
Bonnet J. A., Tracing the calcium phosphorus and iron from a limed and unlimed lateritic soil to the grass and animal blood. Soil Sci. Soc. Am. Proc. 11, 295–297 (1947).
Brown J. C., Iron chlorosis in plants. Adv. Agron. 13, 329–369 (1961).
Brown J. C. and HendricksS. B., Enzymic activities as indications of copper and iron deficiencies in plants. Plant Physiol. 27, 651–660 (1952).
Buehrer T. F., Ariz. Agric. Expt. Sta. Techn. Bull. 42, 154–212 (1932).
Dingra D. R., KanwarJ. S. BhumblaD. R. and SehgalJ. L., Soils of the proposed citrus belt of Punjab. II. Physico-chemical analysis. J. Research (PAU Ludhiana) 2, 154–159 (1965).
Elgala A. M., HamdiH., OmarM. and WafitI., Iron and phosphorus interaction in calcareous soils. II. Effect on chlorosis development and some nutrient element contents in soil and plant. U.A.R.J. Soil Sci. 11. (2), 259–269 (1971).
Hendrickson A. H., A chlorotic condition of pear trees, Proc. Am. Soc. Hort. Sci. 21, 87–90 (1925).
Hodgson J. F., NeeleyK. L. and PusheeJ. C., Iron fertilization of calcareous soils in the green house and laboratory. Soil Sci. Soc. Am. Proc. 36, 320–323 (1972).
Kanwar J. S., Research on trace elements in Punjab-Presentand future. J. Indian Soc. Soil Sci. 12, 221–224 (1964).
Kittrick J. A. and JocksonM. L., Electron microscope observations of the reaction of phosphate with minerals leading to a unified theory of phosphate fixation in soils. J. Soil Sci. 7, 81–89 (1956).
Koening R. A. and JohnsonC. R., Colorimetric determination of phosphorus in biological materials. Industr. Engg. Chem (anal.) 14, 155–156 (1942).
Mann H. B., Availability of manganese and iron as affected by the application of calcium and magnesium carbonate to the soils. Soil Sci. 30, 117–141 (1930).
Sandell E. B., Colorimetric determination of traces of metals. 2nd ed. Interscience Publishers, Inc., New York, 673 p (1950).
Singh C. and Randhawa, N. S., Studies on orchard soils of Bhatinda district. II Distribution of macro and micronutrients. Advances in Food. Held at P.A.U. Ludhiana (1969).
Somers I. I. and ShiveJ. V., The iron manganese relation in plant metabolism. Plant Physiol. 17, 583–602 (1942).
Twyman E. S., The iron and manganese requirements of plants. New Phytol. 50, 210–226 (1951).
Watanabe F. S., LindsayW. L. and OlsonS. R., Nutrient balance involving phosphorus, iron and zinc. Soil. Sci. Soc. Am. Proc. 29, 562–565 (1965).
Wiebosch W. A., Chlorosis in North Holland, its identification and control. Meded. Direct. Tuinb. 10, 556–565 (1947) (Soils and Fertilizers II, 1851).
Willard H. H. and GreathouseL. H., Colorimetric determination of manganese by oxidation with Periodate. J. Am. Chem. Soc. 39, 366 (1917).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Singh, M., Dahiya, S.S. Effect of calcium carbonate and iron on the availability and uptake of iron, manganese, phosphorus and calcium in pea (Pisum sativum L.). Plant Soil 44, 511–520 (1976). https://doi.org/10.1007/BF00011371
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00011371