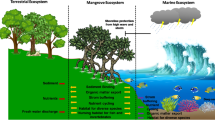
Abstract
Sedimentary bacteria have generally been recognized as an essential food for protists and invertebrates, forming the base of benthic food webs. This trophic role has been well documented, but bacteria play an equally important role as mineralizers of organic detritus and recyclers of essential nutrients. Recent evidence suggests that this latter role is more important than their trophic function in tropical mangrove and coastal sediments. Bacteria in these systems are, on average, more abundant and productive than their counterparts in higher-latitude systems. They account for a disproportionate share of nutrient uptake to the extent that bacterial communities act as a sink for carbon, processing most of the energy and nutrients in tropical aquatic systems. Most bacteria remain unconsumed in tropical deposits, dying naturally and lysing, with the next generation of cells consuming, mineralizing and recycling this material either into new biomass or dissolved material. Bacteria in tropical aquatic sediments are ultimately controlled by inputs of dissolved and particulate detritus, natural mortality and recycling. To replenish damaged ecosystems in the tropics, restoration of the natural geochemical profile in the sediments is necessary to re-initiate the growth of bacteria in order to restore the essential recycling processes which assist in the conservation of nutrients.
Similar content being viewed by others
References
Aller, J. Y. & R. C. Aller, 1986. General characteristics of benthic faunas on the Amazon inner continental shelf with comparison to the shelf off the Changjiang River, East China Sea. Cont. Shelf Res 6: 291–310.
Alongi, D. M., 1988a. Bacterial productivity and microbial biomass in tropical mangrove sediments. Microb. Ecol. 15: 59–79.
Alongi, D. M., 1988b. Microbial-meiofaunal interrelationships in some tropical intertidal sediments. J. Mar. Res. 46: 349–365.
Alongi, D. M., 1989a. Benthic processes across mixed terrigenous-carbonate sedimentary facies on the central Great Barrier Reef continental shelf. Cont. Shelf Res. 9: 629–663.
Alongi, D. M., 1989b. The fate of bacterial biomass and production in marine benthic food chains. In: Hattori, T., Ishida, Y., Maruyama, Y., Molita, R. Y., Uchida, A. (eds), Recent Advances in Microbial Ecology, Japanese Scientific Societies Press, Tokyo: 353–359.
Alongi, D. M., 1990. The ecology of tropical soft-bottom benthic ecosystems. Oceanogr. Mar. Biol. Annu. Rev. 28: 381–496.
Alongi, D. M., 1991. The role of intertidal mudbanks in the diagenesis and export of dissolved and particulate materials from the Fly Delta, Papua New Guinea. J. exp. Mar. Biol. Ecol. 149: 81–107.
Alongi, D. M., 1992. Vertical profiles of bacterial abundance, productivity and growth rates in coastal sediments of the central Great Barrier Reef lagoon. Mar. Biol. 112: 657–663.
Alongi, D. M., K. G. Boto & F. Tirendi, 1989. Effect of exported mangrove litter on bacterial productivity and dissolved organic carbon fluxes in adjacent tropical nearshore sediments. Mar. Ecol. Prog. Ser. 56: 129–140.
Alongi, D. M., P. Christoffersen, F. Tirendi & A. I. Robertson, 1992. The influence of freshwater and material export on sedimentary facies and benthic processes within the Fly Delta and adjacent Gulf of Papua (Papua New Guinea). Cont. Shelf Res. 12: 287–326.
Ayyakkannu, K. & D. Chandramohan, 1971. Occurrence and distribution of phosphate solubilizing bacteria and phosphatose in marine sediments at Porto Novo. Mar. Biol. 11: 201–210.
Blackburn, T. H., 1988. Benthic mineralization and bacterial production In: Blackburn, T. H., Sorensen, J. (eds), Nitrogen Cycling in Coastal Marine Environments, Wiley & Sons, Chichester. 175–190.
Blackburn, T. H., D. Christensen, A. M. Fanger, K. Henriksen, H. Iiyumi, N. Iversen & P. Limpsaichol, 1987. Mineralization processes in mangrove and seagrass sediments. In: Hylleberg, J., (ed.), Ao Yon — a mangrove in the Andaman Sea. Institute of Ecology & Genetics, University of Aarkus, Denmark: 22–32.
Boto, K. G., D. M. Alongi & A. L. J. Nott, 1989. Dissolved organic carbon-bacteria interactions at sediment-water interface in a tropical mangrove system. Mar. Ecol. Prog. Ser. 51: 243–251.
Boto, K. G. & J. T. Wellington, 1983. Phosphorus and nitrogen nutritional status of a northern Australian mangrove forest. Mar. Ecol. Prog. Ser. 11: 63–69.
Christian, R. R. & W. J. Wiebe, 1979. Three experimental regimes in the study of sediment microbial ecology. In: C. D. Litchfield, P. L. Seyfried (eds), Methodology for Biomass Determinations and Microbial Activities in Sediments, Am. Soc. Test. Materials: 148–155.
Clough, B. F., 1988. Conservation and utilisation of mangrove resources. Galaxea 7: 287–296.
Ducklow, H. W., 1990. The biomass, production and fate of bacteria in coral reefs. In: Dubinsky, Z. (ed.) Coral Reefs, Elsevier Sci. Publ., Amsterdam: 265–289.
Dye, A. H., 1983. A method for the quantitative estimation of bacteria from mangrove sediments. Estuar. coast. Shelf Sci. 17: 207–215.
Findlay, R. H., M. B. Trexler, J. B. Guckert & D. C. White, 1990a. Laboratory study of disturbance in marine sediments: response of a microbial community. Mar. Ecol. Prog. Ser. 61: 121–133.
Findlay, R. H., M. B. Trexler & D. C. White, 1990b. Response of a benthic microbial community to biotic disturbance. Mar. Ecol. Prog. Ser. 62: 135–148.
Furtado, J. I., W. B. Morgan, J. R. Pfafllin & K. Ruddle, (eds) 1990. Tropical Resources: Ecology and Development. Harwood Academic Publ., Chur, 306 pp.
Gerlach, S. A., 1978. Food-chain relationships in subtidal silty sand marine sediments and the role of meiofauna in stimulating bacterial productivity Oecologia 33: 55–69.
Hansen, J. A., D. M. Alongi, D. J. W. Moriarty & P. C. Pollard, 1987. The dynamics of benthic microbial communities at Davies Reef, Central Great Barrier Reef, Coral Reefs 6: 63–70.
Hatcher, B. G., R. E. Johannes & A. I. Robertson, 1989. Review of research relevant to the conservation of shallow tropical marine ecosystems. Oceanogr. Mar. Biol. annu. Rev. 27: 337–414.
Herndl, G. J., J. Fagareli, N. Fanuko, P. Peduzzi & V. Turk, 1987. Role of bacteria in the carbon and nitrogen flow between water-column and sediment in a shallow marine bay (Bay of Piran, Northern Adriatic Sea). P.S.Z.N.I. Mar. Ecol. 8: 221–236.
Hoppe, H. G., K. Gocke. D. Zamorano & R. Zimmerman, 1983. Degradation of macromolecular organic compounds in a tropical lagoon (Cienaga Grande, Colombia) and its ecological significance. Int. Revue ges. Hydrobiol. 68: 811–824.
Kemp, P. F., 1988. Bacterivory by benthic ciliates: significance as a carbon source and impact on sediment bacteria. Mar. Ecol. Prog. Ser 49: 163–169.
Kemp, P. F., 1990. The fate of benthic bacterial production. Revue aquat Sci. 2: 109–124.
Kristensen, E., F. O. Andersen & L. H. Kofoed, 1988. Preliminary assessment of benthic community metabolism in a south-east Asian mangrove swamp. Mar. Ecol. Prog. Ser. 48: 137–145.
Kristensen, E., M. Holmer & N. Bussarawit, 1991. Benthic metabolism and sulfate reduction in a south-east Asian mangrove swamp. Mar. Ecol. Prog. Ser. 73: 93–103.
Lathwell, D. J. & T. L. Grove, 1986. Soil-plant relationships in the tropics. Annu Rev. Ecol. Syst. 17: 1–16.
Lewis, W. M., 1987. Tropical limnology. Annu Rev. Ecol. Syst. 18: 159–184.
Matondkar, S. G. P., S. Mahtani & S. Mavinkurve, 1980. Seasonal variations in the microflora from mangrove swamp of Goa. Indian J. mar. Sci. 9: 119–122.
Matondkar, S. G. P., S. Mahtani & S. Mavinkurve 1981. Studies on mangrove swamps of Goa. 1. Heterotrophic bacterial flora from mangrove swamps. Mah. Bull. Natl. Inst. Oceanogr. 14: 325–329.
Meyer-Reil, L. A., 1984. Bacterial biomass and heterotrophic activity in sediments and overlying waters. In: J. E. Hobbie, P. le B. Williams (eds). Heterotrophic activity in the sea, Plenum Press, N.Y.: 523–546.
Moriarty, D. J. W., 1983. Bacterial biomass and productivity in sediments, stromatolites, and water of Hamelin Pool, Shark Bay, Western Australia. Geomicrobiol. J. 3: 121–133.
Moriarty, D. J. W., 1986a. Measurement of bacterial growth rates in aquatic systems from rates of nucleic acid synthesis. Adv. Microb. Ecol. 9: 245–292.
Moriarty, D. J. W., 1986b. Bacterial productivity in ponds used for culture of penaeid prawns. Microb. Ecol. 12: 259–269.
Moriarty, D. J. W., 1989. Relationships of bacterial biomass and production to primary production in marine sediments. In: T. Hattori, Y. Ishida, Y. Maruyama, Y., R. Y. Morita, A. Uchida (eds), Recent Advances in Microbial Ecology, Japanese Scientific Societies Press, Tokyo: 349–354.
Moriarty, D. J. W., 1990. Techniques for estimating bacterial growth rates and production of biomass in aquatic environments. In: J. R. Norris, R. Grigorova (eds), Methods in Microbiology, Vol. 22, Academic Press, London: 211–234.
Moriarty, D. J. W. & P. C. Pollard, 1982. Diel variation of bacterial productivity in seagrass (Zostera capricorni) beds measured by rate of thymidine incorporation into DNA. Mar. Biol. 72: 165–173.
Moriarty, D. J. W., P. I. Boon, J. A. Hansen, W. G. Hunt, I. R. Poiner, P. C. Pollard, G. W. Skyring & D. C. White, 1985. Microbial biomass and productivity in seagrass beds. Geomicrobiol. J. 4: 21–51.
Moriarty, D. J. W., R. Iverson & P. C. Pollard, 1986. Exudation of organic carbon by the seagrass Halodule wrightii and its effect on bacterial growth in the sediment. J. exp. mar. Biol. Ecol. 96: 115–126.
Moriarty, D. J. W., D. G. Roberts & P. C. Pollard, 1990. Primary and bacterial productivity of tropical seagrass communities in the Gulf of Carpentaria, Australia. Mar. Ecol. Prog. Ser. 61: 145–157.
Olah, J., V. R. P. Sinha, S. Ayyappan, C. S. Purushothaman & S. Radheyshyam, 1987. Sediment oxygen consumption in tropical undrainable fish ponds. Int. Revue ges. Hydrobiol. 72: 297–305.
Pollard, P. C. & D. J. W. Moriarty, 1991. Organic carbon decomposition, primary and bacterial productivity and sulphate reduction, in tropical seagrass beds of the Gulf of Carpentaria, Australia. Mar. Ecol. Prog. Ser. 69: 149–159.
Ruess, R. W. & S. J. McNaughton, 1987. Grazing and the dynamics of nutrient and energy-regulated microbial processes in the Serengeti grasslands. Oikos 49: 101–110.
Short, F. T., W. C. Dennison & D. G. Capone, 1990. Phosphorus-limited growth of the tropical seagrass Syringodium filforme in carbonate sediments. Mar. Ecol. Prog. Ser. 62: 169–174.
Singh, R. S., A. S. Raghubanshi, R. S. Singh & S. C. Srivastava, 1989. Microbial biomass acts as a source of plant nutrients in dry tropical forest and savannah. Nature 338: 499–500.
Singh, R. S., S. C. Srivastava, A. S. Raghubanski, J. S. Singh & S. P. Singh, 1991. Microbial C. N and P in dry tropical Savannah: effects of burning and grazing. J. Appl. Ecol. 28: 869–878.
Stanley, S. O., K. G. Boto. D. M. Alongi & F. T. Gillan, 1987. Composition and bacterial utilization of free amino acids in tropical mangrove sediments. Mar. Chem. 22: 13–30.
Tenore, K. R., 1988. Nitrogen in benthic food chains. In: Blackburn, T. H., Sorensen, J. (eds). Nitrogen Cycling in Coastal Marine Environments, Wiley & Sons, Chichester: 191–206.
Tietjen, J. H. & D. M. Alongi, 1990. Population growth and effects of nematodes on nutrient regeneration and bacteria associated with mangrove detritus from northeastern Queensland (Australia). Mar. Ecol. Prog. Ser. 68: 169–180.
Vitousek, P. M. & R. L. Sanford, 1986. Nutrient cycling in moist tropical forest. Annu. Rev. Ecol. Syst. 17: 137–167.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Alongi, D.M. The role of bacteria in nutrient recycling in tropical mangrove and other coastal benthic ecosystems. Hydrobiologia 285, 19–32 (1994). https://doi.org/10.1007/BF00005650
Issue Date:
DOI: https://doi.org/10.1007/BF00005650