Abstract
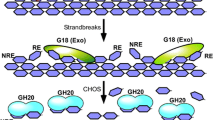
Many plant species accumulate chitinases and ß-1,3-glucanases in response to infection by plant pathogens and to treatments with the plant stress hormone, ethylene. The substrates of these two enzymes, chitin and ß-1,3-glucan, are the main components of the cell walls of most higher fungi. Taken individually, purified chitinases and ß-1,3-glucanases inhibit some fungi but do not affect most of them. However, combinations of the two enzymes inhibit many saprophytic and pathogenic fungi on agar plates or in liquid medium. Microscopic studies indicate that the enzymes attack primarily the hyphal tip. Growing hyphae are highly sensitive when suddenly brought into contact with the antifungal hydrolases. However, they have a potential to adapt and become resistant when exposed continually to the enzymes. This suggests that antifungal hydrolases may be more effective in defense when suddenly brought into contact with invading fungi, e.g. by release from an intracellular compartment, than when present constitutively in the extracellular space.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Arlorio, M., Ludwig, A., Boiler, T., Mischiati, P. and Bonfante-Fasolo, P. (1992) Inhibition of fungal growth by plant chitinases and ß-l,3-glucanases: a morphological study. Protoplasma, in press.
Abeles, F.B., Bosshart, R.P., Forrence, L.E. and Habig, W.H. (1971) Preparation and purification of glucanase and chitinase from bean leaves. Plant Physiol. 47,129–134.
Benhamou, N. and Asselin, A. (1989) Attempted localization of a substrate for chitinases in plant cells reveals abundant N-acetyl-D-glucosamine residues in secondary walls. Biol. Cell 67, 341–350.
Bemasconi, P., Locher, R., Pilet, P.E., Jollès, J. and Jollès, P. (1987) Purification and N-terminal amino acid sequence of a basic lysozyme from Parthenocissus quinquifolia cultured in vitro. Biochim. Biophys. Acta 915, 254–260.
Boiler, T. (1987) Hydrolytic enzymes in plant disease resistance, in T. Kosuge, E.W. Nester (eds.), Plant-Microbe Interactions, Vol. 2, Macmillan, New York, pp. 385–413.
Boiler, T. (1988) Ethylene and the regulation of antifungal hydrolases in plants, in B.J. Miflin (ed.), Oxford Surveys of Plant Molecular and Cell Biology, Vol. 5, Oxford University Press, Oxford, pp. 145–174.
Boiler, T. and Métraux, J.-P. (1988) Extracellular localization of chitinase in cucumber. Physiol. Mol. Plant Pathol. 33,11–16.
Boiler, T. and Vögeli, U. (1984) Vacuolar localization of ethylene-induced chitinase in bean leaves. Plant Physiol. 74, 442–444.
Boiler, T., Gehri, A., Mauch, F. and Vögeli, U. (1983) Chitinase in bean leaves: induction by ethylene, purification, properties, and possible function. Planta 157, 22–31.
Broekaert, W.F., Van Parijs, J., Allen, A.K. and Peumans, W.J. (1988) Comparison of some molecular, enzymatic, and antifungal properties of chitinases from thorn-apple, tobacco, and wheat Physiol. Mol. Plant Pathol. 33, 319–331.
Broekaert, W.F., Van Parijs, J., Leyns, F., Joos, H. and Peumans, W.J. (1989) A chitin-binding lectin from stinging nettle rhizomes with antifungal properties. Science 245,1100–1102.
Broekaert, W.F., Terras, F.R.G., Cammue, B.P.A. and Vanderleyden, J. (1990) An automated quantitative assay for fungal growth inhibiton. FEMS Microbiol. Lett. 69, 55–60.
Broglie, K., Chet, I., Holliday, M., Cressman, R., Biddle, P., Knowlton, S., Mauvis, C.J. and Broglie, R. (1991) Transgenic plants with enhanced resistance to the fungal pathogen Rhizoctonia solani. Science 254,1194–1197.
Côté, F., Cutt, J.R., Asselin, A. and Klessig, D.F. (1991) Pathogenesis-related acidic ß-1,3-glucanase genes of tobacco are regulated by both stress and developmental signals. Mol. Plant-Microbe Interact. 4, 173–181.
Darvill, A.G. and Albersheim, P. (1984) Phytoalexins and their elicitors - a defense against microbial infections in plants. Annu. Rev. Plant Physiol. 35,243–275.
De Jong, A., Cordewener, J., Lo Schiavo, F., Terzi, M., Vandekerckhove, J., Van Kammen, A. and De Vries, S.C. (1992) A carrot somatic embryo mutant is rescued by chitinase. Plant Cell 4,425–433.
Felix, G. and Meins, F., Jr. (1986) Developmental and hormonal regulation of ß-l,3-glucanase in tobacco. Planta 167, 206–211.
Granade, T.C., Hehmann, M.F. and Artis, W.M. (1985) Monitoring of filamentous fungal growth by in situ microspectrophotometry, fragmented mycelium absorbance density, and 14C incorporation: alternatives to mycelial dry weight. Appl. Environ. Microbiol. 49, 101–108.
Grenier, J. and Asselin, A. (1990) Some pathogenesis-related proteins are chitosanases with lytic activity against fungal spores. Mol. Plant-Microbe Interact 3, 401–407.
Ham, K.-S., Kaufmann, S., Albersheim, P. and Darvill, A.G. (1991) Host-Pathogen Interactions XXXIX. A soybean pathogenesis-related protein with ß-l,3-glucanase activity releases phytoalexin elicitor-active heat-stable fragments from fungal cell walls. Mol. Plant-Microbe Interact. 4,545–552.
Inouhe, M. and Nevins, D.J. (1991) Inhibition of auxin-induced cell elongation of maize coleoptiles by antibodies specific for cell wall glucanases. Plant Physiol. 96, 426–431.
Joosten, M.H.A.J. and de Wit, P.J.G.M. (1989) Identification of several pathogenesis-related proteins in tomato leaves inoculated with Cladosporium fulvum (syn. Fulvia fulva) as 1,3-ß-glucanases and chitinases. Plant Physiol. 89, 945–951.
Kaufrmann, S., Legrand, M., Geoffroy, P. and Fritig, B. (1987) Biological function of “pathogenesis-related” proteins: four PR proteins of tobacco have 1,3-ß-glucanase activity. EMBO J. 6, 3209–3212.
Keefe, D., Hinz, U. and Meins, F., Jr. (1990) The effect of ethylene on the cell-type-specific and intracellular localization of ß-l,3-glucanase and chitinase in tobacco leaves. Planta 182, 43–51.
Koide R.T. and Schreiner R.P. (1992) Regulation of the vesicular-arbuscular mycorrhizal symbiosis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 43, 557–581.
Kombrink, E. and Hahlbrock, K. (1986) Resposes of cultured parsley cells to elicitors from phytopathogenic fungi. Timing and dose dependency of elicitor-induced reactions. Plant Physiol. 81, 216–221.
Kombrink, E., Schröder, M., and Hahlbrock, K. (1988) Several “pathogenesis-related” proteins in potato are 1,3-ß-glucanases and chitinases. Proc. Natl. Acad. Sci. USA 85,782–786.
Kunz, C., Ludwig, A., Bertheau, Y. and Boiler, T. (1992) Evaluation of the antifungal activity of the purified chitinase 1 from the filamentous fungus Aphanocladium album. FEMS Microbiol. Lett. 90,105–110.
Lawton, K., Ward, E., Payne, G., Moyer, M. and Ryals, J. (1992) Acidic and basic class III mRNA accumulation in response to TMV infection of tobacco. Plant Mol. Biol. 19, 735–743.
Leah, R., Tommerup, H., Svendsen, I. and Mundy, J. (1991) Biochemical and molecular characterization of three barley seed proteins with antifungal properties. J. Biol. Chem. 266, 1564–1573.
Legrand, M., Kauffmann, S., Geoffroy, P. and Fritig, B. (1987) Biological function of pathogenesis-related proteins: four tobacco pathogenesis-related proteins are chitinases. Proc. Natl. Acad. Sci. USA 84,6750–6754.
Linthorst, H.J.M. (1991) Pathogenesis-related proteins of plants. Crit. Rev. Plant Sci. 10, 123–150.
Lotan, T., Ori, N. and Fluhr, R. (1989) Pathogenesis-related proteins are developmentally regulated in tobacco flowers. Plant Cell 1, 881–887.
Lucas, J., Henschen, A., Lottspeich, F., Vögeli, U. and Boiler, T. (1985) Amino-terminal sequence of ethylene-induced bean leaf chitinase reveals similarities to sugar-binding domains of wheat germ agglutinin. FEBS Lett. 193, 208–210.
Ludwig, A. and Boiler, T. (1990) A method for the study of fimgal growth inhibition by plant proteins. FEMS Microbiol. Lett. 69, 61–66.
Mauch, F. and Staehelin, L.A. (1989) Functional implications of the subcellular localization of ethylene-induced chitinase and ß-l,3-glucanase in bean leaves. Plant Cell 1,447–457.
Mauch, F., Hadwiger, L.A. and Boiler, T. (1984) Ethylene: symptom, not signal for the induction of chitinase and ß-l,3-glucanase in pea pods by pathogens and elicitors. Plant Physiol. 76, 607–611.
Mauch, F., Hadwiger, L.A. and Boiler, T. (1988a) Antifungal hydrolases in pea tissue I. Purification and characterization of two chitinases and two ß-l,3-glucanases differentially regulated during development and in response to fungal infectioa Plant Physiol. 87,325–333.
Mauch, F., Mauch-Mani-B. and Boiler, T. (1988b) Antifungal hydrolases in pea tissue II. Inhibition of fungal growth by combinations of chitinase and ß-l,3-glucanase. Plant Physiol. 88, 936–942.
Meins, F., Jr. and Ahl, P. (1989) Induction of chitinase and ß-l,3-glucanase in tobacco leaves infected with Pseudomonas tabaci and Phytophthora parasitica var. nicotianae. Plant Sci. 61, 155–161.
Meins, F., Jr., Neuhaus, J.-M., Sperisen, C. and Ryals, J. (1992) The primary structure of plant pathogenesis-related glucanohydrolases and their genes, in F. Meins, Jr., and T. Boiler (eds.), Genes Involved in Plant Defense, Springer Verlag, Vienna/New York, pp. 245–282.
Métraux, J.-P., Burkhart, W., Moyer, M., Dincher, S., Middlesteadt, W. Williams, S., Payne, G., Carnes, M. and Ryals, J. (1989) Isolation of a complementary DNA encoding a chitinase with structural homology to a bifunctional lysozyme/chitinase. Proc. Natl. Acad. Sci. USA 86, 896– 900.
Minelman, D., Galun, E., Sharon, N. and Lotan R. (1975) Inhibition of fungal growth by wheat germ agglutinia. Nature 256, 414–416.
Neale, A.D., Wahleithner, J.A., Lund, M., Bonnett, H.T., Kelly, A., Meeks-Wagner, D.R., Peacock, W.J. and Dennis, E.S. (1990) Chitinase, ß-l,3-glucanase, osmotin and extensin are expressed in tobacco explants during flower formation. Plant Cell 2, 673–684.
Neuhaus, J.-M., Ahl-Goy, P., Hinz, U., Flores, S. and Meins, F., Jr. (1991a) High-level expression of a tobacco chitinase gene in Nicotiana sylvestris. Susceptibility of transgenic plants to Cercospora nicotianae infection. Plant Mol. Biol. 16,141–151.
Neuhaus, J.-M., Sticher, L., Meins, F., Jr. and Boiler, T. (1991b) A short C-terminal sequence is necessary and sufficient for the targeting of chitinases to the plant vacuole. Proc. Natl. Acad. Sci. USA 88,10362–10366.
Payne, G., Ahl, P., Moyer, M., Harper, A., Beck, J., Meins, F., Jr. and Ryals, J. (1990a) Isolation of complementary DNA clones encoding pathogenesis-related proteins P and Q, two acidic chitinases from tobacco. Proc. Natl. Acad. Sci. USA 87, 98–102.
Payne, G., Ward, E., Gaffhey, T., Ahl-Goy, P., Moyer, M., Harper, A., Meins, F., Jr. and Ryals, J. (1990b) Evidence for a third structural class of ß-l,3-glucanase in tobacco. Plant Mol. Biol. 15, 797–808.
Pegg, G.F. and Young, D.H. (1982) Purification and characterization of chitinase enzymes from healthy and Verticilliwn albo-atrwn-Mected tomato plants, and from Verticillium albo-atrum. Physiol. Plant Pathol. 21,389–409.
Roberts, W.K. and Selitrennikoff, C.P. (1988) Plant and bacterial chitinases differ in antifungal activity. J. Gea Microbiol. 134, 169–176.
Schlumbaum, A., Mauch, F., Vögeli, U. and Boiler, T. (1986) Plant chitinases are potent inhibitors of fungal growth. Nature 324, 365–367.
Schröder, M., Hahlbrock, K. and Kombrink, E. (1992) Temporal and spatial patterns of 1,3-ß-glucanase and chitinase induction in potato leaves infected by Phytophthora infestans. Plant J. 2,161–172.
Shinshi, H., Mohnen, D. and Meins, R, Jr. (1987) Regulation of a plant pathogenesis-related enzyme: inhibition of chitinase and chitinase mRNA accumulation in cultured tobacco tissues by auxin and cytokinin. Proc. Natl. Acad. Sci. USA 84, 89–93.
Shinshi, H., Wenzler, H., Neuhaus, J.-M., Felix, G., Hofsteenge, J. and Meins, F., Jr. (1988) Evidence for N- and C-terminal processing of a plant defense-related enzyme. Primary structure of tobacco prepro-ß-l,3-glucanase. Proc. Natl. Acad. Sci. USA 85, 5541–5545.
Shinshi, H., Neuhaus, J.-M., Ryals, J. and Meins, F., Jr. (1990) Structure of a tobacco endochitinase gene: evidence that different chitinase genes can arise by transposition of sequences encoding a cysteine-rich domain. Plant Mol. Biol. 14, 357–368.
Taiz, L. (1984) Plant cell expansion: regulation of cell wall mechanical properties. Annu. Rev. Plant Physiol. 35, 585–657.
Takeuchi, Y., Yoshikawa, M., Takeba, G., Tanaka, K., Shibata, D. and Horino, O. (1990) Molecular cloning and ethylene induction of mRNA encoding a phytoalexin elicitor-releasing factor, ß-l,3-endoglucanase, in soybean. Plant Physiol. 93,673–682.
van den Bulcke, M., Bauw, G., Castresana, C., van Montagu, M., Vanderkerckhove, J. (1989) Characterization of vacuolar and extracellular ß-l,3-glucanases of tobacco: evidence for a strictly compartmentalized plant defense system. Proc. Natl. Acad. Sci. USA 86, 2673–2677.
Van Parijs, J., Broekaert, W.F., Goldstein, I.J. and Peumans, W.J. (1991) Hevein: an antifungal protein from rubber-tree (Hevea brasiliensis) latex. Planta 183, 258–264.
Verburg, J.G. and Huynh, Q.K. (1991) Purification and characterization of an antifungal chitinase from Arabidopsis thaliana. Plant Physiol. 95,450–455.
Vögeli, U., Meins, F., Jr. and Boiler, T. (1988) Co-ordinated regulation of chitinase and ß-1,3- glucanase in bean leaves. Planta 174, 364–372.
Vögeli-Lange, R., Hansen-Gehri, A., Boiler, T. and Meins, F., Jr. (1988) Induction of the defense related glucanohydrolases, ß-l,3-glucanase and chitinase, by tobacco mosaic virus infection of tobacco leaves. Plant Sci. 54, 171–176.
Ward, E.R., Payne, G.B., Moyer, M.B., Williams, S.C., Dincher, S.S., Sharkey, K.C., Beck, J.J., Taylor, H.T., Ahl-Goy, P., Meins, F., Jr. and Ryals, J.A. (1991) Differential regulation of ß-1,3-glucanase messenger RNAs in response to pathogen infection. Plant Physiol. 96, 390–397.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 1993 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Boller, T. (1993). Antimicrobial Functions of the Plant Hydrolases, Chitinase and ß-1,3-Glucanase. In: Fritig, B., Legrand, M. (eds) Mechanisms of Plant Defense Responses. Developments in Plant Pathology, vol 2. Springer, Dordrecht. https://doi.org/10.1007/978-94-011-1737-1_124
Download citation
DOI: https://doi.org/10.1007/978-94-011-1737-1_124
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-010-4761-6
Online ISBN: 978-94-011-1737-1
eBook Packages: Springer Book Archive