Abstract
In current time, the crowd of depression has increased rapidly across colleges and universities in China. They often suffer anhedonia, social failure, drug abuse and so on. The recent reports points out that the brain reward system showed damaged in patients with depression, so the identification of the dysfunction in the brain reward system is really crucial. By analyzing brain magnetic resonance imaging (MRI) data, this article aimed to identify the gray matter volume (GMV) abnormalities in brain reward system between first-episode medication-naive patients with major depressive disorder (MDD) and healthy controls (HCs). 14 medication-naive participants with MDD aged 19–24 years (6 males, 8 females) and 18 healthy controls aged 19–24 years (9 males, 9 females) were recruited. We used voxel-based morphometry (VBM) to analyze brain imaging data. Then, two sample t-test was applied to detect GM abnormalities in MDD compared to HCs. This study found that MDD showed increased gray matter volume (GMV) in putamen, precuneus and amygdala in right hemisphere compared to HCs. Furthermore, MDD showed decreased GMV in left rectus, right orbital medial prefrontal cortex (omPFC), left superior temporal gyrus (STG) and left insula compared to HCs. However, no significant changes were found in caudate nucleus. These experimental results show that the depression disorder causes extensive damage in reward system and subcortical brain regions, and the alterations in the rectus, the omPFC, STG, precuneus and amygdala, may be characters of MDD in first episode.
Similar content being viewed by others
Keywords
- Major depressive disorder
- Reward system
- Gray matter volume
- Magnetic resonance imaging
- Voxel-based morphometry
1 Introduction
MDD is a psychological disorder that dose harm to human health. It has become the most common mental disease in the world with the higher and higher morbidity [33]. According to a recently published research [31], the number of depressive patients accounts for about a quarter of all diseases in the world and will be the second most prevalent disorder by 2030. People suffering from depressive disorders experience substantial loss of quality of life, including anhedonia [1], social failure, drug abuse, and more severely suicide [9]. Especially, anhedonia is a major symptom of depression characterized by losing the abilities and motivations to experience the joy of happiness or enjoy themselves from usual activities. It is estimated that approximately 60% of all suicides have a history of depression [20]. Not only did depression cause severe damage to the patient’s quality of life, but also it brought huge economic pressure on family and health care industry at the same time.
With the development and innovation of the neuroimaging technologies, such as MRI, the researches of MDD have been entering a new level, and the researches on the reward loop were explored more and more deeply [4]. The reward loop, also known as the limbic system dopamine reward circuit which considered to be associated with depression, is a neural network consisting of the nucleus accumbens, caudate nucleus, putamen, thalamus, hypothalamus, amygdala and other deep brain nuclei and medial prefrontal cortex. A paper published recently generalized the findings about the reward loop [8], implying that the cortical-basal ganglia circuit is at the heart of the reward system. It emphasized that the key structures in this circuit are the anterior cingulate cortex (ACC), the orbital frontal cortex (OFC), the ventral striatum, the ventral pallidum, and the midbrain dopamine neurons. Furthermore, some structure, involving the amygdala, hippocampus and specific brainstem regions also play significant role in rewarding [23]. In addition, most studies have demonstrated functional lesions in the reward network in depressed patient using functional MRI in last few years. Particularly, there were hyporesponsivity in striatal reward regions, frontal cortex and cingulate gyrus in MDD [28].
In this paper we choose structural MRI to explore the relationship between the dysfunction of the reward system and GMV abnormalities. Although the roles of these key regions are found, the exact pattern of the GM abnormalities in the operation of reward in depression is not defined. For instance, the striatum, as the core component of reward circuits, showed inconsistent phenomenon. Macmaster suggested that no differences were found in the caudate in adolescent major depressive disorder and bipolar depression [16]. On the other hand, depressed adolescents had smaller gray matter volume relatively to HCs in the frontal lobe and caudate nucleus bilaterally in Shad’s report [26]. Although most studies have found reduction of GMV in OFC and ACC and have consistency in the function of these regions [2, 7], Stratmann revealed discrepant findings that no differences existed between controls and MDD individuals [29]. Certain studies showed reduction of GMV in the amygdala [5], however others didn’t find any changes in amygdala or larger volume in this area [7]. In addition, the inconsistency of hippocampus structural abnormality was also showed in previous studies. Chen and his colleagues reported decreased GMV in hippocampus [5]. Whereas, there were researches failed to detect any micro GMV differences between controls and patients in this area [6]. This kind of heterogeneity is widespread in the pathophysiology of MDD. The reasons that contribute to inconsistent and discrepant results are complicated. First, participants at various age levels are implied cross studies and the limited small number of subjects account for the variability of structural brain volume changes. Second, the usage of analytic methods may have impact on the observations of structural change in brain regions. Furthermore, illness duration and number of depressive episodes cause an intrinsic heterogeneity of studies regarding for the brain structural abnormalities in depressed patients.
On the basis of the discoveries explored by previous brain structural literature, we aimed to investigate the relationship between GMV alterations and damage in reward network in a cohort of young and well-characterized adults who are college students aged between 19 and 24 by using whole-brain analysis methods. Considering that medication has an effect on the GMV in depressed patients, this paper employed medication-naive participants undergoing MRI scanning to obtain a better result. Furthermore, because reward activities and emotion are regulated by circuits including most brain regions, such as PFC, especially in OFC, ACC, striatum areas and limbic system [8, 22], we hypothesize that medication-free patients of first-episode MDD may have GMV alterations especially in OFC, ACC, striatum and amygdala areas.
2 Materials and Methods
2.1 Participants
14 medication-naive participants with MDD aged 19–24 years (6 males, 8 females) and 18 healthy controls aged 19–24 years (9 males, 9 females) were recruited. All participants were recruited from the School of Information at Dalian Maritime University from 2013 to 2016. Both patients and controls were paid a small honorarium for study participation. This study was approved by the local Ethics Committee and all participants provided written informed consent.
2.2 Magnetic Resonance Imaging Acquisition
The MRI studies were conducted with a 3.0 Tesla Siemens Trio MRI scanner in Affiliated Xinhua Hospital of Dalian University. Acquisition was performed using an 8-channel head coil. Three-dimensional T1-weighted anatomical images were acquired using a 3D SPGR sequence (\(TR = 1680\,\text {ms}; TE = 285\,\text {ms}; Flip Angle = 9; TI = 500\,\text {ms}; NEX = 1; ASSET = 1.5; \) Frequency direction: S/I). A total of 160 contiguous 1 mm slices were acquired with a \(384 \times 384\) matrix, with an in-plane resolution of 1 mm\(\,\times \,\)1 mm resulting in isotropic voxels.
2.3 Statistical Analysis
SPM8 (The FIL Methods group, UK) and VBM8 (Structural Brain Mapping Group, Germany) software programs were used in both preprocessing and processing for scanned images based on MATLAB R2011a. Brain volume normalizing, bias correcting and segmentation into gray matter, white matter, and cerebrospinal fluid were performed using VBM8 toolbox. VBM8 toolbox is based on an optimized voxel-based morphometric protocol that helps to increase the signal to noise ratio. After preprocessing, SPM8 was used to smooth the images. In the whole-brain analyses, we performed a two sample t-test in the SPM8 to determine whether there were any significant regional GMV differences between MDD and healthy controls. A threshold of \(p<0.05\) uncorrected for the whole-brain volume at a cluster level was used to select statistically significant brain regions whose \(cluster size>10 \).
3 Results
3.1 Group Differences in Brain GM Volumes
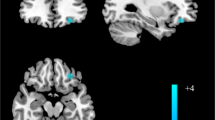
The VBM results showed that the gray matter density in the left rectus, the right omPFC, left STG, left insula, left ACC, left putamen nucleus and left hippocampus was significantly lower in the first episode of severe depression than in the normal control group (\(p <0.05\)). The density of gray matter in the right precuneus, right amygdala and left thalamus was significantly higher than that in the normal control group (\(p <0.05\), Table 1, Figs. 1, 2 and 3).
Regions of gray matter volume decreases among patients with MDD as compared with HCs. (A) Gray matter volume reduced in the region of left rectus and right orbital medial frontal gyrus (peak MNI coordinate: Rectus_L: \(0, 63, -15; t=5.2\), Frontal_Med_Orb_R: \( 1.5, 63, -13.5; t= 4.94, p<0.05)\). (B) Gray matter volume reduced in the region of left superior temporal gyrus (peak MNI coordinate: \( -61.5, -18, 4.5; t= 4.20, p<0.05\)). (C) Gray matter volume reduced in the left insula (peak MNI coordinate: \( -21, -21, -12; t= 2.23, p<0.05\)). The color density represents the Tscore.
Regions of gray matter volume decreases mildly among patients with MDD as compared with HCs. (D) Gray matter volume reduced in the region of left anterior cingulum (peak MNI coordinate: \(-9, 27, -10.5; t= 2.75, p<0.05)\). (E) Gray matter volume reduced in the region of left putamen nucleus (peak MNI coordinate: \(-30, -15, 1.5; t= 2.66, p<0.05\)). (F) Gray matter volume reduced in the left hippocampus (peak MNI coordinate: \(-30, -25.5, 18; t= 3.27, p<0.05\)). The color density represents the Tscore.
Regions of gray matter volume increases among patients with MDD as compared with HCs. (A) Gray matter volume increased in the region of right precuneus (peak MNI coordinate: \(10.5, -43.5, 43.5; t= 4.4., p<0.05\)). (B) Gray matter volume increased in the right amygdala (peak MNI coordinate: \( 27, 4.5, -15; t= 3.43, p<0.05\)). (C) Gray matter volume increased in the right thalamus (peak MNI coordinate: \(-1.5,-15, 16.5; t= 2.99, p<0.05\)). The color density represents the Tscore.
4 Discussion
In the current study, we compared the GMV in reward system of depression patients and healthy subjects in a cohort of young and well-characterized adults who are medication-free college students aged between 19 and 24 by using whole-brain analysis methods, ruling out the effects of medication and aging, finding that the abnormal changes of GMV in depressive patients were bidirectional. Areas with smaller GMV in depression group than control group mainly located in the left rectus, the omPFC and the left STG, on the other hand, brain regions with significantly increased GMV are the precuneus and the amygdala in right hemisphere and the left thalamus. However, no alterations were found in caudate nucleus. The appearance of this phenomenon indicated that depressive disorder caused widespread structural abnormalities on reward circuits and fronto-limbic network [2, 22], which plays key roles in the regulation of reward processing and emotion, and the significant alteration in the left rectus, right omPFC, STG, precuneus and amygdala and the slight variations in putamen nucleus and ACC may contribute to the dysfunction of rewarding and emotion in first-episode medication-naive MDD.
The left rectus and the right omPFC, which are located in the orbital frontal areas, are the most obvious areas of reduction regions and this phenomenon was consistent with our hypothesis. In a review of reward network, it emphasized that the omPFC plays a key role in the reward anticipation, such as sensory rewards and abstract rewards (e.g., money), especially near the gyrus rectus about the latter activation [8]. Moreover, the PFC, interacting with some limbic regions, also makes great contributions to the emotion processing and cognition in humans brain [25]. A meta-analysis study of depression focused on investigating brain structural changes in the cortico-striatal-pallidal-thalamic circuits, in which the PFC, especially the orbital PFC showed significantly high level of reduction of GMV. Accordingly, they approved that GMV declining in this neural circuits of depressed patients might play a significant role in the dysfunctional reward processing in depression [2]. Scheuerecker combined structural and functional researches about depressive disorders and found that decreased volume in OPFC in depressed patients by using the VBM toolkit [25]. They came to a conclusion that the OFPC is a key brain area in the emotional circuits and the alteration of the OFPC structure results in functional changes of the emotional circuits. The experimental results of the present study supported the crucial role of the OFPC in the regulation of reward, emotion and cognition and confirmed that structural changes would lead to functional changes, and furthermore hinder the expression of normal feeling.
In the current study, the putamen nucleus which is the core of the reward network and the ACC had slight GMV reduction. The putamen, critical link of the cortico-striatal-pallidal-thalamic circuits, interconnecting the cerebral cortex and subcortical areas, efficiently transmit and process emotional and rewarding information [2], and many fMRI researches stressed lower activation in response to rewarding stimulus [28]. Lai explored GMV of subcortical regions in first-episode medication-naive depressive patients and indicated that the patients had smaller volumes of the left putamen nucleus [12]. Bora et al. also suggested lower GMV in the putamen [2]. Our results about ACC were consistent with previous studies [2, 32]. ACC, especially dorsal ACC, has a variety of functions, involving motivation, cognition, and motor control. Notably, the vital duty of the ACC is to monitor these functions in complex conditions [8]. Green research discovered that MDD participants showed decreased activation of the rostral cingulate gyrus during reward selection and anticipation [28], and this study supported the core role of ACC in reward system. A paper of mild depressive patients reported reduced gray matter volume in the ACC and orbitofrontal cortex using VBM analysis [32]. The reason why the extents of GMV decrease in these areas were moderate might be that the depressive participants in the current study were in the initial stage. Secondly, all subjects were in the transition period of youth to the middle-aged, eliminating the effect of aging [17]. Furthermore, education also should be taken into consideration. These discoveries indicated that the slightly decreased GMV in putamen and ACC in first-episode medication-free MDD relative to HCs might be a mark of dysfunction in reward system.
The directions of the volume changes in hippocampus and amygdala are not consistent in present study that shows decreased GMV in hippocampal and increased GMV in the amygdala. Studies of the hypothalamic-pituitary-adrenal axis (HPA) have shown that the HPA axis may have different effects on the hippocampus and the amygdala [18]. Chronic stress or trauma experiences may aggravate the burden of stress regulation and make increased activation of HPA, which lead to amounts of release of cortisol. Excessive cortisol caused two diametrically opposite effects on the amygdala and hippocampus, hypertrophy of the amygdala while atrophy of the hippocampus. This theory directly supports our results. Li studied the relationship between the severity of depression and the hippocampus and amygdala, found that the volume of amygdaloid nucleus in the mild depression group significantly enlarged and the hippocampal volume decreased in the patients with depression according to the severity of the disease (mild, moderate, major), especially in major group [15]. The participants in our experiments were college students, whose severity of depression were not so high, therefore, mild reduction of hippocampal volume and relatively more evident enlarged volume in amygdala were observed. Consequently, it might mean that overmuch stress could cause abnormalities of the amygdala and hippocampus, and gradually, their dysfunction would lead to negative emotions.
The masses of regional GMV loss in left superior temporal gyrus (STG) occurred in present study. Previous articles demonstrated the function of this region in the perception and emotion recognition and also highlighted its heavy connections with face- and body-specific cortical and subcortical structures [3]. Its interrelation with frontal-limbic circuits underpins human emotion cognition. Substantial GMV loss was present in the left STG, which is also in consistent with prior studies [13]. A meta-analysis of first-episode patients with depression revealed a significant reduction of GMV in the temporal gyrus using both the pooled meta-analysis and the subgroup meta-analysis [34]. Our findings of GMV loss in this brain region replicate previous results. With respect to this, the alterations in left STG might be a common presence in the early stage of depression.
There was also volume reduction in insula in our findings. As a key area of the limbic system, the insula has extensive connections with other brain regions about emotion processing, such as the OPFC, the ACC and the hippocampus. Ho found significantly decreased functional connectivity between insula and ACC in MDD relative to healthy controls when evaluating negative emotional stimuli [10]. Hence, the destruction of the insular function will cause tremendous damage to people’s emotions, cognition, feelings and so on. Stratmann Mhad carried out experiments with more than 200 subjects and found a significant reduction of GMV in the anterior insula, and the longer the illness duration was, the more obvious the differences between groups would be [29]. Our findings that GMV reduction in insula in depressed patients were consistent with the previous articles [32]. Taken previous researches into consideration, the GMV reduction in insula may be a representative phenomenon in the course of depression. These abnormalities in the PFC, STG, insula and ACC support the notion that MDD could be conceived as a network dysfunction in brain primarily.
In this study, we also found that the GMV of precuneus was increased in depressive group compared to healthy subjects. As a core component of the default mode network (DMN), the roles of precuneus encompasses autobiographical memory retrieval, reward outcome monitoring, and emotional stimulus processing [30]. Abnormal functional connectivity in precuneus had been reported in cognitive network [27] and processing of negative stimuli [10]. Li et al. demonstrated enlarged GMV of precuneus in young women with subthreshold depression in comparison to healthy controls [14]. Another structural imaging study also reported increased gray matter density in precuneus among first-episode antipsychotic-naive MDD [35]. So that, this result may indicate hyperactivity of the precuneus maybe aggravate the symptoms of depression in MDD.
The thalamus, subcortical regions involved in the brain reward system, is a relay station for the input information in the cerebral cortex [19], actively sending message to the cortex in various mechanisms [24]. A study of single episode, medication-free MDD subjects demonstrated structural abnormalities of frontal-subcortical circuits in the early stage of MDD, such as increased GMV in left thalamus, indicating that this abnormal phenomenon may be a critical component in the mechanism of depression and excluding the influence of the medication treatment [11]. Another study about first-episode patients with MDD also concluded the unusual state in the thalamus with increased GMV [36]. A recently published article about medication-naive patients with first-episode depression also represented the similar results [21]. Accordingly, we predicted that the thalamus might play an essential role in the pathology of depression and the larger GMV in this region in MDD could be a general status.
The other areas in striatum, such as caudate, didn’t show significant changes of GMV in present study. The results of previous studies on this region were not consistent. Numerous papers found reduced GMV in caudate [2, 26], however no differences were detected in Macmaster research [16]. There might be several reasons. First, the durations of depression state in MDD were short in present study, and lesions of patients were not obvious. Next, analytic methods and parameters defined in this study might contribute to current result to some extent. Accordingly, more researches are needed to identify the inconsistency.
The limitations of the present study deserve mention. First, the small sample size limited the generalizability of the results. In the next stage, we should employ more participants to investigate the subtle variation between groups. Second, our cohort were college students, whose brains are undergoing GM loss during normal aging [17], affecting the comparative result to a certain extent. Furthermore, the results may not apply to older cohorts, so further researches about MDD in other age groups need to be done. Finally, other elements, such as sex and severity of depression, should be taken into consideration to precisely explore the mechanism of depression. Additionally, functional MRI, DTI and structural MRI should be study together to obtain more valuable information.
In conclusion, we demonstrated that college patients with first-episode depression, who were medication-naive, showed widespread abnormal structural GMV changes in cortical and subcortical brain areas which involved in reward circuits, especially in OPFC, amygdala, STG and precuneus. The structural abnormalities might mark medication-naive MDD in first episode. In addition to these significant variations, we also identified slight GMV alterations in other brain areas in reward system, such as putamen, ACC, thalamus and hippocampus, which may be somewhat helpful in discerning MDD of first episode.
References
Admon, R., Pizzagalli, D.A.: Dysfunctional reward processing in depression. Curr. Opin. Psychol. 4, 114–118 (2015)
Bora, E., Harrison, B.J., Davey, C.G., Yucel, M., Pantelis, C.: Meta-analysis of volumetric abnormalities in cortico-striatal-pallidal-thalamic circuits in major depressive disorder. Psychol. Med. 42(4), 671–681 (2012)
Candidi, M., Stienen, B.M., Aglioti, S.M., De, G.B.: Virtual lesion of right posterior superior temporal sulcus modulates conscious visual perception of fearful expressions in faces and bodies. Cortex 65, 184–194 (2015)
Chen, L., Zhang, W., Liu, H., Feng, S., Chen, C.L.P., Wang, H.: A space affine matching approach to fMRI time series analysis. IEEE Trans. NanoBiosci. 15(5), 468–480 (2016). https://doi.org/10.1109/TNB.2016.2572401
Chen, V.C., Shen, C.Y., Liang, S.H., Li, Z.H., Tyan, Y.S., Liao, Y.T., Huang, Y.C., Lee, Y., Mcintyre, R.S., Weng, J.C.: Assessment of abnormal brain structures and networks in major depressive disorder using morphometric and connectome analyses. J. Affect. Disord. 205, 103–111 (2016)
Finkelmeyer, A., Nilsson, J., He, J., Stevens, L., Maller, J.J., Moss, R.A., Small, S., Gallagher, P., Coventry, K., Ferrier, I.N.: Altered hippocampal function in major depression despite intact structure and resting perfusion. Psychol. Med. 46(10), 2157–2168 (2016)
Grieve, S.M., Korgaonkar, M.S., Koslow, S.H., Evian, G., Williams, L.M.: Widespread reductions in gray matter volume in depression. Neuroimage Clin. 3, 332–339 (2013)
Haber, S.N., Knutson, B.: The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 35(1), 4–26 (2010)
Henderson, S.E., Johnson, A.R., Vallejo, A.I., Katz, L., Wong, E., Gabbay, V.: A preliminary study of white matter in adolescent depression: relationships with illness severity, anhedonia, and irritability. Front. Psychiatry 4, 152 (2013)
Ho, T.C., Yang, G., Wu, J., Cassey, P., Brown, S.D., Hoang, N., Chan, M., Connolly, C.G., Henje-Blom, E., Duncan, L.G.: Functional connectivity of negative emotional processing in adolescent depression. J. Affect. Disord. 155(3), 65–74 (2014)
Kong, L., Wu, F., Tang, Y., Ren, L., Kong, D., Liu, Y., Xu, K., Wang, F.: Frontal-subcortical volumetric deficits in single episode, medication-naive depressed patients and the effects of 8 weeks fluoxetine treatment: A VBM-DARTEL study. Plos One 9(1), e79055 (2014)
Lai, C.H.: Hippocampal and subcortical alterations of first-episode, medication-naive major depressive disorder with panic disorder patients. J. Neuropsychiatry Clin. Neurosci. 26(2), 142–149 (2014)
Lai, C.H., Wu, Y.T.: The gray matter alterations in major depressive disorder and panic disorder: putative differences in the pathogenesis. J. Affect. Disord. 186, 1–6 (2015)
Li, H., Wei, D., Sun, J., Chen, Q., Zhang, Q., Jiang, Q.: Brain structural alterations associated with young women with subthreshold depression. Scientific Reports 5, 9707 (2015)
Li, Y., Yan, J., Wang, D., Sun, M., Zhu, Y., Zhu, X., Jiang, P., Yin, R., Zhao, L.: Magnetic resonance study of the structure and function of the hippocampus and amygdala in patients with depression. Chin. Med. J. 127(20), 3610–3615 (2014)
Macmaster, F.P., Carrey, N., Langevin, L.M., Jaworska, N., Crawford, S.: Disorder-specific volumetric brain difference in adolescent major depressive disorder and bipolar depression. Brain Imag. Behav. 8(1), 119–127 (2014)
Manard, M., Bahri, M.A., Salmon, E., Collette, F.: Relationship between grey matter integrity and executive abilities in aging. Brain Res. 1642, 562–580 (2016)
Pagliaccio, D., Luby, J.L., Bogdan, R., Agrawal, A., Gaffrey, M.S., Belden, A.C., Botteron, K.N., Harms, M.P., Barch, D.M.: Stress-system genes and life stress predict cortisol levels and amygdala and hippocampal volumes in children. Neuropsychopharmacology 39(5), 1245–1253 (2014)
Parnaudeau, S., O’Neill, P.K., Bolkan, S.S., Ward, R.D., Abbas, A.I., Roth, B.L., Balsam, P.D., Gordon, J.A., Kellendonk, C.: Inhibition of mediodorsal thalamus disrupts thalamofrontal connectivity and cognition. Neuron 77(6), 1151–1162 (2013)
Peng, H., Wu, K., Li, J., Qi, H., Guo, S., Chi, M., Wu, X., Guo, Y., Yang, Y., Ning, Y.: Increased suicide attempts in young depressed patients with abnormal temporal-parietal-limbic gray matter volume. J. Affect. Disord. 165, 69–73 (2014)
Peng, W., Chen, Z., Yin, L., Jia, Z., Gong, Q.: Essential brain structural alterations in major depressive disorder: a voxel-wise meta-analysis on first episode, medication-naive patients. J. Affect. Disord. 199, 114–123 (2016)
Price, J.L., Drevets, W.C.: Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn. Sci. 16(1), 61–71 (2012)
Russo, S.J., Nestler, E.J.: The brain reward circuitry in mood disorders. Nat. Rev. Neurosci. 14(9), 609–625 (2013)
Saalmann, Y.B., Kastner, S.: Cognitive and perceptual functions of the visual thalamus. Neuron 71(2), 209–223 (2011)
Scheuerecker, J., Meisenzahl, E.M., Koutsouleris, N., Roesner, M., SchPf, V., Linn, J., Wiesmann, M., Bruckmann, H., MeLler, H.J., Frodl, T.: Orbitofrontal volume reductions during emotion recognition in patients with major depression. J. Psychiatry Neurosci. 35(5), 311–320 (2010)
Shad, M.U., Muddasani, S., Rao, U.: Gray matter differences between healthy and depressed adolescents: a voxel-based morphometry study. J. Child Adolesc. Psychopharmacol. 22(3), 190–197 (2012)
Shen, T., Li, C., Wang, B., Yang, W.M., Zhang, C., Wu, Z., Qiu, M.H., Liu, J., Xu, Y.F., Peng, D.H.: Increased cognition connectivity network in major depression disorder: a FMRI study. Psychiatry Invest. 12(2), 227–234 (2015)
Smoski, M.J., Felder, J., Bizzell, J., Green, S.R., Ernst, M., Lynch, T.R., Dichter, G.S.: fMRI of alterations in reward selection, anticipation, and feedback in major depressive disorder. J. Affect. Disord. 118(1–3), 69 (2009)
Stratmann, M., Konrad, C., Kugel, H., Krug, A., Schoning, S., Ohrmann, P., Uhlmann, C., Postert, C., Suslow, T., Heindel, W.: Insular and hippocampal gray matter volume reductions in patients with major depressive disorder. Plos One 9(7), e102692 (2014)
Utevsky, A.V., Smith, D.V., Huettel, S.A.: Precuneus is a functional core of the default-mode network. J. Neurosci. Official J. Soc. Neurosci. 34(3), 932–940 (2014)
Vavakova, M., Durackova, Z., Trebaticka, J.: Markers of oxidative stress and neuroprogression in depression disorder. Oxidative Med. Cell. Longevity 2015(2), 898393 (2015)
Webb, C.A., Weber, M., Mundy, E.A., Killgore, W.D.: Reduced gray matter volume in the anterior cingulate, orbitofrontal cortex and thalamus as a function of mild depressive symptoms: a voxel-based morphometric analysis. Psychol. Med. 44(13), 2833–2843 (2014)
Wjh, P.B., Yuri, M., Femke, L., Nicole, V.: Understanding the somatic consequences of depression: biological mechanisms and the role of depression symptom profile. BMC Med. 11(1), 129 (2013)
Zhang, H., Li, L., Wu, M., Chen, Z., Hu, X., Chen, Y., Zhu, H., Jia, Z., Gong, Q.: Brain gray matter alterations in first episodes of depression: a meta-analysis of whole-brain studies. Neurosci. Biobehav. Rev. 60, 43–50 (2016)
Zhang, J., Xiao, J., Zhu, X., Wang, X., Yao, S.: Voxel-based morphometry on grey matter concentration of the brain in first-episode, antipsychotic-naive major depressive disorder. J. Central South Univ. (Medical Science) 36(4), 307–311 (2011)
Zhang, X., Yao, S., Zhu, X., Wang, X., Zhu, X., Zhong, M.: Gray matter volume abnormalities in individuals with cognitive vulnerability to depression: a voxel-based morphometry study. J. Affect. Disord. 136(3), 443–452 (2012)
Acknowledgment
This work is partly supported by the National Natural Science Foundation of China (Grant Nos. 61472058 and 61772102).
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this paper
Cite this paper
Qi, Q. et al. (2018). Gray Matter Volume Abnormalities in the Reward System in First-Episode Patients with Major Depressive Disorder. In: Hassanien, A., Tolba, M., Elhoseny, M., Mostafa, M. (eds) The International Conference on Advanced Machine Learning Technologies and Applications (AMLTA2018). AMLTA 2018. Advances in Intelligent Systems and Computing, vol 723. Springer, Cham. https://doi.org/10.1007/978-3-319-74690-6_69
Download citation
DOI: https://doi.org/10.1007/978-3-319-74690-6_69
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-74689-0
Online ISBN: 978-3-319-74690-6
eBook Packages: EngineeringEngineering (R0)