Abstract
In order to provide references for heavy metals diagnosis of Vetiver grass, Raman spectroscopy technology was employed, the partial least squares (PLS) quantitative analysis model of heavy metal lead of Vetiver grass root was established, different processing methods were used to optimize the Raman spectra of Vetiver grass roots, Successive projection algorithm (SPA) was applied to screen the bands of Raman spectrum and enhance the model’s accuracy. The best Raman spectroscopy quantitative analysis model of lead content in Vetiver grass root were set up, 20 unknown samples were used to test the quality of optimized model. The prediction correlation coefficient and the root mean square error were 0.607 and 0.040 g/kg, respectively.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
As a kind of not easily biodegradable toxic substances, heavy metals are harmful to the vast majority of biology, especially the human. However, heavy metals can be gradually accumulated in plants and animals by the food chains [1]. Ingestion of heavy metals in agricultural products is one of the important factors that cause the accumulation of heavy metal in human body [2]. Therefore, the content of heavy metals in foods is an important index for food safety risk assessment. In order to reduce the harm caused by eating crops, which containing excessive heavy metals, seeking a kind of rapid and nondestructive method for detecting heavy metal content in plants is necessary.
However, the traditional methods for detecting heavy metal content in plants, including X-ray fluorescence spectroscopy (XRF) [3], inductively coupled argon (ICP) [4] and atomic absorption spectroscopy (AAS) [5] and so on are always combined with digestion and chemical analysis instrument, which are time-consuming and plant-damage [6]. Raman spectroscopy, which is a spectrum analysis technology with the advantages of fast, non-destructive, non-pretreatment, has the board application prospect in terms of detecting heavy metal contents in plants indirectly [7]. Although the pure heavy metals cannot be estimated directly by Raman spectroscopy, heavy metals in plants usually combined with the organic molecular groups that have Raman spectral information [8]. Therefore heavy metals in plants can be indirectly detected by using spectral technique basing on the chelation.
Vetiver grass can live in the range of −22 °C to 55 °C temperature, which has strong vitality. It also can grow up in the environment of heavy metal pollution, shows the strong tolerance of heavy metals [9, 10]. Because of these characteristics, Vetiver grass becomes one of the ideal plants for soil and water conservation and phytoremediation of soil heavy metal pollution [11].
2 Materials and Methods
2.1 Samples and Reagents
The Vetiver grasses in this study were collected in the heavy metal pollution fields of Guixi city, China. The ultrapure water was produced by ultrapure water polishing system (TS-RO-10L/H, TAOSHI Water Equipment Engineering Co. Ltd., China), with 18.2 MΩ cm resistivity. All chemical reagents (HNO3, HClO4) in this study are of analytical purity grade, and purchased from Sinopharm chemical reagent Co. Ltd., China.
2.2 Samples Preparation
The vetiver grass were washed by tap water until removing the soil and dust on the surface of plants, and rinsed by ultrapure water twice in order to remove the interference of heavy metals in water. Then the roots of vetiver grass were cut by scissors for the samples for Raman spectroscopy detection.
2.3 Raman Spectral Measurement
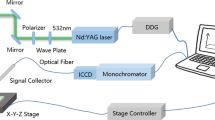
All Raman spectra were measurement from 90 cm−1 to 3500 cm−1 with a confocal microprobe Raman spectrophotometer (SENTERRA, Bruker, Germany). Each root sample of Vetiver grass was put on the quartz glass slide, which has been put on the sample frame of spectrophotometer. Eyepiece of 10 times and objective lens of 50 times were employed respectively. In order to make the laser focus on the internal organizations of Vetiver grass roots, fine tune the sample frame until the clearest images of plant surface was observed.
At the same time, the impact to organizations of Vetiver grass roots by the quantity of heat of laser should be considered. Although the higher power laser can improve the spectrum information, it also can be more easily destroy the organizations, which would influence the outcome of Raman spectral measurement. Choose the optimal instrument experimental parameters through several tests to ensure that the plant in the laser focus will not lead to excessive heat structure of plant tissue damage: the excitation wavelength was 785 cm−1, the Resolution was 0.5 cm−1, the integration time was 5 s, the light spot diameter was 50*1000 microns, and the power of laser was 10 mw.
In order to reduce the spectral measurement error, each root sample of Vetiver grass was measured three times, so each spectrum of root sample of Vetiver grass was averaged from three parallel measurements.
2.4 Measurement of Quality Parameters
All root samples of Vetiver grass were dried to constant weight by drying oven (DHG-9101-19A, Shanghai Hong Electronic Technology Co. Ltd., China) with temperature of 75 to 80 centigrade after Raman spectral measurement, then ground into powder respectively with a pulverizer.
Each Vetiver grass root powder sample was weighed 0.2 g by electronic balance, soaked with 10 ml HNO3 in the flask and sealed overnight, then digested by an electric hot plate (Mb, Beijing Kewei Yongxing Instrument Co. Ltd., China) until the large granular sediments were nearly disappeared. The flask was removed with the crucible tongs for cooling, 10 mL HNO3 and 5 mL HClO4 standard solution were added into the flask respectively after cooling, then heated by electric hot plate again until white smoke appeared in the flask. Removing the flask for cooling again, the sample solution in the flask was washed into 25 ml volumetric flask by deionized water, and then filtered into another 25 mLvolumetric flask. Finally, flame atomic absorption spectrometer (AA280FS, Varian, America) was used for measuring the total lead in solution of Vetiver grass roots.
3 Results and Discussion
3.1 Raman Spectral Features of Vetiver Grass Root
All Raman spectrums of Vetiver grass root samples were averaged to be a mean Raman spectrum, and shown in Fig. 1. Because of the low power of laser, the Raman signal of Vetiver grass roots is very weak, the Raman peak are not obviously. What’s more, the weak Raman characteristic peaks were covered up by the interfering substance in the plants and the fluorescence effect should be another main reason for causing this phenomenon.
Different wave number Raman characteristic peaks represent the position of chemical bonds, and also reflect the corresponding molecular structure information. So the information of some molecules in Vetiver grass root, which have strong Raman effects, can be found according to the figure of the Raman spectrum peak position, and shown in Table 1. The result shows that the alkanes, hydrocarbons and ketone compounds in Vetiver grass root have stronger Raman information than the other compounds.
At the same time, in the range of 2000 to 3500 cm−1 of Raman spectrum of Vetiver grass root, the effective Raman characteristic information in Raman spectra of Vetiver grass root does not exist. Therefore, in order to reduce the interference from other useless information, excluding this region of Raman spectrum, 150~2020 cm−1 spectral region are selected for establishing Raman mathematical analysis model of Vetiver grass root.
3.2 Reference Analysis
In order to facilitate the model’s modeling and testing, 60 plants were randomly selected in 80 Vetiver grass samples as a calibration set, the remaining 20 plants as prediction sets. The statistical analyses including range, mean and standard deviation (S.D.) for lead values in Vetiver grass roots are performed in Table 2. It shows that the heavy metal lead values are within the wide range for the samples of Vetiver grass roots, which are beneficial to produce robust and stable calibration models and evaluate the models’ predictive ability.
3.3 Quantitative Models of Vetiver Grass Root
Because of the weak Raman signal and the strong fluorescence intensity, a lot of Raman signal usually are covered up by the fluorescence background. Therefore, in order to establish a great Raman mathematical analysis model, enlarging the effective information in the Raman spectra and enhancing the signal-to-noise ratio of spectrum are indispensable. Different spectral preprocessing methods can effectively eliminate some interference information because of their different functions in spectrum optimization. In this study, six spectral preprocessing methods, smoothing, standard normal variate (SNV), multiplicative scatter correction (MSC), first derivative, second derivative and baseline correction, were employed to optimize the quality of models. The quantitative partial least squares (PLS) models of different preprocessed spectrums were established with Unscrambler 8.0 software package, the PLS model of raw spectrum was also established for the sake of contrastive analysis, and shown in Table 3.
From the contrastive analysis of cross-validation correlation coefficients (rcv) and cross-validation root mean square errors (RMSECV) in the Table 3, it can be found that two preprocessing methods (1st derivative, 2nd derivative) have the ability to optimize the spectrum information of Vetiver grass roots, with the higher Rcv and lower RMSECV than the raw spectrum. The 2nd derivative is the best preprocessing method for optimizing PLS model. However, the rest four preprocessing methods can not raise the quality of model, it should be reasonable. The main functions of derivative preprocessing method are distinguishing the overlapping peaks and improving the resolution of spectrum. It shows that the important interference factors of model established are overlapping peaks.
3.4 Identification of Optimum Wavelength
Each wave number of spectrum means the corresponding information of tested object. Some noises may have been collected by Raman spectrophotometer when the raman spectrums of Vetiver grass roots were collected. In order to reduce the negative influence of noises in the spectrum, eliminating the spectral ranges of noises and extracting the wavelengths of target component is necessary.
Successive projections algorithm (SPA) [12] is a forward selection method, it can promote the quality of model and ensure the realizability of model. Therefore, SPA has been widely praised in the multiple qualitative analysis of the spectrum of applications. With the help of Matlab R2010a software package, SPA was employed to solve the problem of wavelength selection after spectral preprocessing.
It can be seen from Fig. 2, the prediction root mean square error RMSE of model is changed along with the number of variables. In order to select the lowest prediction root mean square error, the number of selected variables of 8 was chose for establishing model. The corresponding PLS model parameters of Pb content in Vetiver grass roots was shown in Table 4.
Table 4 shows that the effective variables of model was screened by SPA, the cross-validation correlation coefficients (rcv) was rise from 0.675 to 0.697, the cross-validation root mean square errors (RMSECV) was decreased from 0.034 g/kg to 0.033 g/kg, and the principal component number (PCs) was changed to 8. Therefore, the algorithm of SPA can improve the model quality of detecting lead content in Vetiver grass roots, but the effect is not obvious.
In order to find the compounds chelated with heavy metal lead in Vetiver grass roots, the characteristics of Raman spectra wavelengths screened by SPA were listed in Table 5, it shows that a wide variety of compounds in Vetiver grass roots may be chelated with lead, including normal alkanes, bromides, chloroalkane, allenes, hydrazone and so on.
3.5 Prediction of Quality Attributes with the Optimum Wavelength
In order to verify the feasibility of the Pb detecting models in Vetiver grass roots, external validation is further done with the 20 validation samples. The Raman scattering intensities corresponding wave numbers screened by SPA were intercepted for the variables of models, the atomic absorption values were served as measured values, and the optimized PLS models were used for prediction. Figure 3 shows the relationships of the predicted and the measured values of the validation samples, the correlation coefficients of prediction (rp) and root mean square errors of prediction (RMSEP) of Vetiver grass root are 0.607 and 0.040 g/kg, respectively.
4 Conclusion
The calibration and validation statistics obtained in this study showed the potential of Raman spectroscopy to predict heavy metal Pb in the root of Vetiver grass. Although the accuracy of model with this method was lower than the routine analysis, Raman spectroscopy could be used as a fast and simple tool to diagnose the content of heavy metal Pb in Vetiver grass approximately. Overall, Raman spectroscopy can be used as a reference method for diagnosing the content of heavy metal Pb in Vetiver grass, but the accuracy needs to be improved.
References
Devkota, B., Schmidt, G.H.: Accumulation of heavy metals in food plants and grasshoppers from the Taigetos Mountains, Greece. Agric. Ecosyst. Environ. 78(1), 85–91 (2000)
Arora, M., Kiran, B., Rani, S., Rani, A., Kaur, B., Mittal, N.: Heavy metal accumulation in vegetables irrigated with water from different sources. Food Chem. 111(4), 811–815 (2008)
Donner, E., Howard, D.L., Jonge, M.D.D., Paterson, D., Cheah, M.H., Naidu, R., Lombi, E.: X-ray absorption and micro X-ray fluorescence spectroscopy investigation of copper and zinc speciation in biosolids. Environ. Sci. Technol. 45(17), 7249–7257 (2011)
Tormen, L., Torres, D.P., Dittert, I.M., Araújo, R.G., Frescura, V.L., Curtius, A.J.: Rapid assessment of metal contamination in commercial fruit juices by inductively coupled mass spectrometry after a simple dilution. J. Food Compos. Anal. 24(1), 95–102 (2011)
Tufekci, M., Bulut, V.N., Elvan, H., Ozdes, D., Soylak, M., Duran, C.: Determination of Pb(II), Zn(II), Cd(II), and Co(II) ions by flame atomic absorption spectrometry in food and water samples after preconcentration by coprecipitation with Mo(VI)-diethyldithiocarbamate. Environ. Monit. Assess. 185(2), 1107–1115 (2013)
Zhen, X.S., Lu, A.H., Gao, X., Zhao, J., Zheng, D.S.: Contamination of heavy metals in soil present situation and method. Soil Environ. Sci. 11(1), 79–84 (2002)
Torreggiani, A., Domènech, J., Atrian, S., Capdevila, M., Tinti, A.: Raman study of in vivo synthesized Zn(II)- metallothionein complexes: structural insight into metal clusters and protein folding. Biopolymers 89(12), 1114–1124 (2008)
Tan, W.N., Li, Z.A., Zou, B.: Molecular mechanisms of plant tolerance to heavy metals. J. Plant Ecol. 30(4), 703–712 (2006)
Pang, J., Chan, G.S.Y., Zhang, J., Liang, J., Wong, M.H.: Physiological aspects of vetiver grass for rehabilitation in abandoned metalliferous mine wastes. Chemosphere 52(9), 1559–1570 (2003)
Dalton, P.A., Smith, R.J., Truong, P.N.V.: Vetiver grass hedges for erosion control on a cropped flood plain: hedge hydraulics. Agric. Water Manag. 31(1–2), 91–104 (1996)
Datta, R., Das, P., Smith, S., Punamiya, P., Ramanathan, D.M., Reddy, R., Sarkar, D.: Phytoremediation potential of vetiver grass [chrysopogon zizanioides (L.)] for tetracycline. Int. J. Phytorem. 15(4), 343–351 (2013)
Ghasemi-Varnamkhasti, M., Mohtasebi, S.S., Rodriguez-Mendez, M.L., Gomes, A.A., Araújo, M.C.U., Galvão, R.K.: Screening analysis of beer ageing using near infrared spectroscopy and the Successive Projections Algorithm for variable selection. Talanta 89, 286–291 (2012)
Acknowledgements
This project is supported by the National Natural Science Foundation of China (61178036, 31160250), Financial support was provided by the National High Technology Research and Development Program of China (863program) (no. 2012aa101906), Ganpo excellence project 555 Talent Plan of Jiangxi Province (2011-64), Center of Photoelctric Detection Technology Engineering of Jiangxi Province (2012-155), Graduate Science and Technology Innovation Fund projects of Jiangxi Province (YC 2013-S157).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 IFIP International Federation for Information Processing
About this paper
Cite this paper
Liu, Y., Zhang, Y., Jiang, L., Wang, H. (2016). Determination of Lead (Pb) Content in Vetiver Grass Roots by Raman Spectroscopy. In: Li, D., Li, Z. (eds) Computer and Computing Technologies in Agriculture IX. CCTA 2015. IFIP Advances in Information and Communication Technology, vol 478. Springer, Cham. https://doi.org/10.1007/978-3-319-48357-3_29
Download citation
DOI: https://doi.org/10.1007/978-3-319-48357-3_29
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-48356-6
Online ISBN: 978-3-319-48357-3
eBook Packages: Computer ScienceComputer Science (R0)