Abstract
As with most archipelagos, geography played a central role in the assembly and evolution of the endemic-rich biological communities of the Gulf of Guinea oceanic islands. The islands are located at moderate distances from the species-rich African continent that surrounds them to the east and north. This proximity facilitated colonization by many branches of the tree of life, but gene flow between the islands and continent was low enough that many lineages evolved in isolation once they reached the archipelago, resulting in many endemic species. Furthermore, several of the island taxa belong to groups typically considered to be “poor dispersers” across sea barriers, which strongly supports a role for natural rafts in seeding the islands. Oceanic currents, including the freshwater pathways that extend from large river drainages into the Gulf of Guinea during the rainy season, also support this hypothesis. The distances between the islands are equivalent to those between the islands and the continent such that inter-island dispersal events appear to be relatively rare and thus few taxa are shared between them. Still, the islands present multiple cases of secondary contact leading to hybridization and genetic introgression between closely related lineages—providing several models to study the role and consequences of gene flow in evolution. Most taxa for which molecular estimates of divergence time have been derived are much younger than the ages of the islands. This pattern is consistent with high species turnover, likely resulting from a combination of small island sizes, proximity to the African continent and a long history of intense volcanic activity. The Gulf of Guinea oceanic islands provide multiple examples of classical adaptations to island life (the “island syndrome”), including giants and dwarves, ornament and color loss, among others. In addition, emerging studies of birds are highlighting the importance of competition regimes in driving phenotypic change—with examples of both character release (low inter-specific competition) and character displacement (inter-specific competition upon secondary contact). Collectively, the Gulf of Guinea oceanic islands offer unique opportunities to study adaptation and speciation in a range of taxa and contexts.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Islands, and oceanic islands in particular, have always occupied a special place in human imagination (cf. Schalansky 2010). Their isolation and well-defined borders make them worlds apart, microcosms often populated by unique and peculiar creatures. For the naturalist, oceanic islands are one of the most fruitful settings for the study of evolutionary and ecological processes, including adaptation, speciation, and community assembly (Losos and Ricklefs 2009; Whittaker et al. 2017). Charles Darwin hinted at islands serving as “natural laboratories” in his account of the Beagle expedition, after having visited the Galapagos in September 1835 (Darwin 1845: 377–378). It was Alfred Russel Wallace, however, who put it clearly in his fundamental work aptly named Island Life under the section Importance of Islands in the Study of the Distribution of Organisms (Wallace 1880: 234):
In islands we have the facts of distribution often presented to us in their simplest forms, along with others which become gradually more and more complex; and we are therefore able to proceed step by step in the solution of the problems they present. (…) [W]hen we have mastered the difficulties presented by the peculiarities of island life we shall find it comparatively easy to deal with the more complex and less clearly defined problems of continental distribution.
The depiction of islands as natural laboratories arises from their inherent simplicity (systems with well-defined boundaries, generally small, and with a depauperate biota), together with being striking centers of evolutionary change and diversification by virtue of their isolation and specific environmental constraints (Wallace 1880; Carlquist 1965; Grant 1998a; Emerson 2002; Losos and Ricklefs 2009; Whittaker et al. 2017). Both these characteristics are particularly evident in the spectacular radiations of the most isolated island systems, as illustrated by the Hawaiian Drosophila in which up to 1000 species may have evolved from just one or two species (Carson and Kaneshiro 1976; Kaneshiro et al. 1995). Correspondingly, much progress on the study of evolution on islands has stemmed from remote islands such as the Hawaiian archipelago (Wagner and Funk 1995; Craddock 2000) and the Galápagos (Grant and Grant 2008). More recently, it has become clear that island systems closer to continents have the potential to significantly advance our understanding of the processes driving diversification.
As one approaches the mainland, the complexity of island systems increases as dispersal and gene flow between the mainland and the island populations become more frequent (cf. Fig. 1.1 in Whittaker 1998). “Intermediate island systems” are those archipelagos that lie between remote islands systems, virtually independent from the mainland, and systems so close to the mainland that speciation in situ is not possible (Melo 2007; Ricklefs and Bermingham 2007). Intermediate island systems are promising natural laboratories as they may provide a simple setting to investigate the role of gene flow in evolution, which remains a question of fundamental importance in evolutionary biology (Dowling and Secor 1997; Seehausen 2004; Pinho and Hey 2010; Feder et al. 2012; Abbott et al. 2013; Buerkle 2014; Arnold 2015; Taylor and Larson 2019; Matute and Cooper 2021). In addition, the faunas of intermediate island systems are typically derived from several distinct families (independent replicates for evolutionary studies), rather than being dominated by a few species-rich genera that have adaptively radiated. Consequently, patterns of community assembly in such archipelagos are likely more similar to those of continents than are those of more isolated archipelagos where most of the diversity is derived from a few extensive radiations (Melo 2007; Ricklefs and Bermingham 2007).
Here we provide an overview of the biogeography and evolution of the biota of the oceanic islands of the Gulf of Guinea (Príncipe, São Tomé, and Annobón), highlighting their potential to advance our understanding of the processes generating diversity: from population divergence to speciation to community assembly. In addition, this setting provides several excellent models to study the role of gene flow with respect to the evolution of divergent phenotypes and patterns of genome-wide differentiation. Readers will likely notice that this overview is limited and biased towards terrestrial vertebrates. For other taxonomic groups, the diversity of the islands is still incompletely documented and described (Ceríaco et al. 2022a), and thus building the essential foundation for future hypothesis-driven studies is still a work in progress for these other taxa. A brief overview of marine biogeography in the Gulf of Guinea is described in Costa et al. (2022).
Biogeography
The Importance of Geography
The updated checklists for terrestrial groups in the oceanic islands of the Gulf of Guinea (reference list in Ceríaco et al. 2022a) reveal a few principal patterns: (1) high levels of endemism; (2) wide representation across the tree of life; (3) in situ radiations are rare and result in few species; and (4) the biological communities of each island are largely unique, with few endemics shared between them. With the exception of the Odonata (dragonflies and damselflies), which have relatively low species diversity in the Gulf of Guinea and just one endemic species (Dijkstra and Tate 2022), the three islands have some of the highest concentrations of endemic species in the world for several groups, including mosquitoes (Loiseau et al. 2019), amphibians (Bell et al. 2022), terrestrial reptiles (Ceríaco et al. 2022b), and birds (Melo et al. 2022). These patterns are particularly remarkable in relation to the small size of these islands (just over 1000 km2 combined) and are likely a consequence of their favorable geographic setting.
Just in the Right Place: Close, But Not Too Close, to a Large and Species-Rich Continent
The diversity of unique genera and families in the archipelago across taxonomic groups from fungi to frogs is indicative of a large number of colonizations from the mainland. High levels of endemism, once again across many taxonomic groups, indicate that many of these island colonizers have subsequently diverged from their mainland counterparts. The islands are therefore close enough to the continent to receive a diverse array of mainland dispersers but far enough away for these to diverge once they arrive to the islands. These are two defining traits of intermediate island systems: the likelihood of colonizations is higher than on remote systems and the conditions for population divergence are preserved (Melo 2007; Ricklefs and Bermingham 2007). For groups such as birds, where the concentration of island endemics is the highest in the world (Melo et al. 2022), it is as if the oceanic islands of the Gulf of Guinea are located at the perfect distance from the mainland to optimize the balance between colonization (as a source of new lineages) and isolation (reduced gene flow to allow for genetic differentiation). Such an optimal distance will likely vary among taxonomic groups according to their dispersal potential.
The adjacent African continent also hosts the species-rich Congolian rainforests and the Guinean Forests of the West Africa biodiversity hotspot (IUCN 2015), which surround the islands to the east and north, respectively. Thus, the islands are proximal to extensive coastlines of a large and biodiverse landmass. Being situated adjacent to a large landmass is the most important predictor of global plant species diversity on islands (Weigelt and Kreft 2013). In addition, the islands and much of the adjacent mainland share similar habitats (notably rainforest), which increases the chance of successful establishment following sweepstakes dispersal events (Weigelt and Kreft 2013).
Colonization Outweighs In Situ Diversification as a Source of New Species Diversity
Independent colonizations from the mainland, rather than in situ diversification, is the dominant process by which species diversity accumulated in the archipelago (Box 6.1). For example, the 29 endemic bird species present on the three oceanic islands derive from 20 to 22 separate colonizations from the mainland, and only three are shared between islands (Melo et al. 2022). Of the 164 endemic vascular plant taxa of the Gulf of Guinea islands only a small subset is shared between more than one island, and an even more diminutive number is shared with Bioko (Figueiredo 1994; Stévart et al. 2022). Likewise, all nine amphibian species are endemic to a single island, and five are the sole representative of their family on their respective island (Bell et al. 2022). Dispersal between islands, and subsequent isolation, has also been a source of further endemic species in some vertebrate groups including Hyperolius reed frogs (Bell et al. 2015a, b), Hemidactylus geckos (Miller et al. 2012), Trachylepis skinks (Ceríaco et al. 2016), Boaedon house snakes (Ceríaco et al. 2021) and birds, of which the five-species radiation of Zosterops white-eyes is the best example (Melo et al. 2011, 2022). However, for most organisms, each island is closer to an independent unit rather than part of a tight-knit archipelago.
The extensive evolutionary radiations described from remote archipelagos are facilitated by inter-island dispersal events within the archipelago followed by divergent adaptation to fill open ecological niches (Schluter 2000; Gillespie et al. 2020). In the case of the oceanic islands of the Gulf of Guinea, the dearth of adaptive radiation may be a consequence of low inter-island colonization (due to the large distances that separate them), limited opportunities to adapt to novel ecological space (due to the high phylogenetic and ecological diversity of colonizers from the mainland; Schluter 2000; Ricklefs and Bermingham 2007; Rundell and Price 2009; Gillespie et al. 2020), and reduced dispersal ability of island species (Box 6.2).
Box 6.1 Speciation on Oceanic Islands
The most detailed model of how populations diversify on oceanic islands was proposed more than 100 years ago by the British naturalist Robert C. L. Perkins (Grant 2000, 2001). His “archipelago radiation model” (Perkins 1913) was derived from his work on the radiation of Hawaiian honeycreepers (Aves: Fringillidae; Perkins 1901), and subsequently strengthened with his studies of other Hawaiian vertebrates (Perkins 1903) and insects (Perkins 1913). In Perkin’s archipelago radiation model (Panel 3 of figure below), speciation is initiated by population subdivision (isolation: allopatry), which leads to selection-driven divergence, potentially aided by random factors (the concept of drift had not yet been formulated). In a second stage, populations diverging in isolation may meet on the same island (secondary contact: sympatry). They may either interbreed, obscuring the divergence and merging into a single population once more, or they may co-exist as increasingly distinct entities even if some interbreeding occurs. In this case, competition between the two populations will lead to further divergence resulting in ecological character displacement. In other words, the most similar individuals of each species suffer the strongest competition, such that extreme phenotypes are favored by selection and intermediate phenotypes are selected against (Brown and Wilson 1956; Grant 1972). Two things are surprising regarding this model: (1) how complete and specific it is and (2) how it was forgotten by most evolutionary biologists in the ensuing decades (Grant 2000, 2001). The deep insights of Perkins, including the central role of competition in driving phenotypic divergence, are particularly impressive considering that his archipelago radiation model is very similar to the “ecological speciation model” proposed in the twenty-first century (Rundle and Nosil 2005; Nosil 2012).

Pathways for speciation in oceanic archipelagos—using the Gulf of Guinea as an example. In this schematic, the single endemic species on Annobón has always arisen by allospeciation
In many archipelagos, speciation is achieved by divergence in isolation (allospeciation: Mayr and Diamond 2001), the first step of the radiation model (Panels 1 and 2 of figure above). This may even be the case for most archipelagos, as recently confirmed for birds in a global analysis of diversification on islands (Valente et al. 2020). It may also be deemed the most passive, or trivial, speciation mode as permanently isolated populations will always follow distinct evolutionary paths. Phenotypic diversification is often limited in this setting, especially when the mainland and island provide similar habitats. At the other extreme, populations may diverge and speciate fully in sympatry (Panel 4 of figure above; sympatric speciation). This mechanism is not included in Perkins’ model and although much work has been devoted to demonstrating speciation without any sort of geographic isolation, it has been extremely difficult to find convincing cases in nature (Coyne and Orr 2004; Coyne 2007; Bolnick and FitzPatrick 2007). Often, even the best candidates have experienced an initial period of isolation—even a short one—just as in the archipelago radiation model (Feder et al. 2003; Martin et al. 2015a).
The composition of the community across an archipelago can reveal which of the above scenarios played a major role in the origin of endemic species. For instance, the number of families represents the minimum number of colonizations from the mainland, whereas single representatives from mainland groups provide unambiguous cases of allospeciation. The presence of sympatric congeneric endemic species in an archipelago indicates the groups where molecular-based studies are needed, as such species could be the result of any of the speciation modes. There are several such instances in the Gulf of Guinea oceanic islands that have not yet been investigated with molecular data including among several groups of plants (Garcia and Shevock 2022; Stévart et al. 2022), mollusks (Panisi et al. 2022), mushrooms (Desjardin and Perry 2022), arachnids (Crews and Esposito 2022), and insects Mendes and Bivar-de-Sousa 2022; Nève et al. 2022). An important limitation of molecular studies, however, is that they cannot account for the possibility that undetected extinctions have removed the true sister species of extant species. In the volcanic islands of the Gulf of Guinea, no suitable fossil ground has yet been found and hence the extent to which extinctions may confound our inferences of speciation mode is unknown.
Getting There: Modes of Active and Passive Dispersal
Although the location of the oceanic islands of the Gulf of Guinea is favorable to colonization from the continent, it is still a considerable distance to travel for many organisms. The task is made easier for active flyers, such as birds, bats, or large insects. For organisms that disperse passively in the air—the aerial plankton—the dominant wind currents determine the most likely sources of colonizers. In the Gulf of Guinea, these are the southwestern monsoon winds, responsible for the high precipitation that sustains the rainforests, and the northern dry harmattan winds (Ceríaco et al. 2022c). It is the meeting of these two air masses that determines the position of the meteorological equator. The southwestern winds are unlikely to have dispersed colonizers from continental Africa but may have promoted inter-island dispersal from the southwest to the northeast (i.e., Annobón to São Tomé to Príncipe). Passive wind-dispersing organisms are therefore more likely to have originated from West Africa, to the north of the archipelago, and this is indeed the case for angiosperms (Exell 1973) and Simuliidae black-flies (Mustapha et al. 2006). Dispersal via the northern harmattan winds is likely to have been more prominent during glacial cycles, when they displaced the meteorological equator further south (Lézine et al. 1994) and thus extending further into the Gulf. These hypotheses regarding both the direction, timing, and periodicity of colonization for wind-dispersed taxa can be tested with molecular phylogenetic analyses.
Ocean currents determine the most likely sources of aquatic organisms and of terrestrial organisms that disperse in water, including some seed plants and marine fishes that became secondarily adapted to the freshwater bodies of the islands (Costa et al. 2022). These currents have also likely played a major role in facilitating the arrival of many non-volant, non-swimming, and salt-intolerant animals via passive dispersal on floating vegetation rafts (e.g., Ali and Fritz 2021). These include all amphibians (Bell et al. 2022), several fossorial reptiles (Ceríaco et al. 2022b), and the two species of endemic shrews (Rainho et al. 2022). Likewise, plants with low dispersal ability also likely reached the islands via rafting, for instance, the unusual plant Sciaphila ledermannii, a mycoheterotroph that obtains its nutrients by a symbiotic relationship with fungi and lacks obvious structures for either wind or animal dispersal of its seeds (Daniel 2010). Other species that likely reached the islands in this way include species pairs with tight ecological associations unlikely to be maintained by independent dispersal events, such as the hypothesized simultaneous colonization of São Tomé by the sheet-web tarantula Allothele and its kleptoparasitic spider Isela (Charles Griswold, pers. comm.).
Several factors come together in the Gulf of Guinea to support the rafting hypothesis (Measey et al. 2007; Ceríaco et al. 2022c). First, three large rivers (from north to south: Niger, Ogooué, and Congo) drain into the Gulf of Guinea in the vicinity of the islands. These rivers are among the largest in the world with exceptional freshwater discharge during the rainy season. Second, tropical Africa receives very high precipitation levels during the rainy season that can lead to occasional downfalls of river margins, resulting in rafts of vegetation and soil—a necessary requisite for different groups of animals and plants, such as fossorial amphibians and reptiles that made it to the islands (Bell et al. 2022; Ceríaco et al. 2022b). During the rainy season, high freshwater discharge leads to a large outflow of the rivers into the sea, creating extensive freshwater plumes that create a superficial low-salinity layer in the sea (Richardson and Walsh 1986; Jourdin et al. 2006), and protect the rafts from the intrusion of saltwater. Finally, the dominant ocean currents in the Gulf of Guinea direct the freshwater plumes of the Niger and Congo rivers towards the islands. In summary, the ocean currents together with the top layer of freshwater create “freshwater paths” that carry floating natural rafts from West and Central African river drainages towards the islands (Measey et al. 2007).
The dispersal histories of island species can be inferred using molecular data to build phylogenies and identify the most closely related species or populations on the continent. For instance, a phylogeographic approach revealed that the Gray Parrot Psittacus erithacus first reached Príncipe Island from West Africa (c. 1 Ma ago), and that in contemporary times new colonizers arrived from Central African populations (Melo and O'Ryan 2007). By contrast, phylogenetic studies of the endemic São Tomé caecilians suggest they are the product of a single dispersal event (c. 1 Ma ago) from East Africa (Loader et al. 2007). Likewise, the House Snakes Boaedon are the product of a single dispersal event to the archipelago from Southern Africa (Ceríaco et al. 2021). The rare instances of inter-island dispersal can be a bit more challenging to decipher from molecular data (e.g., Melo et al. 2011). Such studies will typically require population-level genetic sampling to estimate patterns of genetic diversity for each island (e.g., Bell et al. 2015b; Weinell et al. 2019) with the assumption that each successive colonization event from the source population represents a bottleneck, leading to sequential reductions in genetic diversity along the island chain (Clegg et al. 2002). Thus far, such biogeographic studies have primarily been conducted for many of the islands’ amphibians, reptiles, and birds with results supporting instances of dispersal from West, Central, East, and Southern Africa, as well as some cases of inter-island dispersal (Bell et al. 2022; Ceríaco et al. 2022b; Melo et al. 2022). As more phylogenetic and population genetic studies become available from a greater diversity of taxa, we will start to gain a better understanding of the dominant continent-to-island and inter-island dispersal pathways and likely mechanisms.
The Temporal Setting
The oceanic islands of the Gulf of Guinea originated from the activity of the Cameroon Volcanic Line, which began c. 30 my ago (Burke 2001). As such they are a relatively old island system, with the age of Príncipe estimated at 31 Ma, São Tomé at 15 Ma, and Annobón at 6 Ma (Ceríaco et al. 2022c). For comparison, the ages of the islands of the Hawaiian archipelago range from 5 to 0.5 Ma (Carson and Clague 1995) and the Galapagos from less than 0.5 to 3 Ma (Harpp and Geist 2018). Thus, in theory, the islands of the Gulf of Guinea have had extensive evolutionary time to accumulate species diversity and endemism. The tempo at which species richness has accrued, however, may have been influenced by many global climatic factors (e.g., changes in sea level and exposed coastline of the African continent), as well as local geological factors (e.g., devastating volcanic eruptions). As the fossil record in the region is poor, molecular clock approaches can be employed to estimate divergence times between the island endemics and their closest mainland relatives. In turn, these dated phylogenies can be used to infer the corresponding colonization time frame for a given lineage (Fig. 6.1) and reconstruct the timeline of community assembly.
The colonization date of an archipelago lies between the time of most ancient common ancestor (MACA) and the time of most recent common ancestor (MRCA). Time of MACA assumes that the appearance of the insular lineages was simultaneous with colonization. Time of MRCA does not take into account post-colonization demographic effects on genetic diversity. Even if the true MACA and/or MRCA went extinct (or were not sampled) they would be located within this interval. Adapted from Vences (2005) and Hayward and Stone (2006)
The currently available divergence dating studies suggest that the old age of the islands has not necessarily translated to an abundance of very old lineages. In plants, although afromontane paleo-endemics are present, these co-exist with a large assemblage of neo-endemics (Figueiredo 1994). Likewise, for other groups, most molecular estimates of divergence times indicate that many endemics are much younger than the ages of the islands. For example, from 22 divergence time estimates for endemic birds, 18 occurred within the last 2 Ma, 3 within the last 3–5 Ma, and the oldest dates to 8 Ma (Table 24.1 in Melo et al. 2022). The endemic fruit fly Drosophila santomea (Llopart et al. 2002; Turissini and Matute 2017), caecilians (Loader et al. 2007), and shrews (Nicolas et al. 2019) also represent recent colonization and speciation events within the last 2 Ma, while inferred colonization dates for reed frogs (Bell et al. 2015a), the ridged frog Ptychadena newtoni (Zimkus et al. 2017), and skink Trachylepis thomensis (Weinell et al. 2019), date to the Late-Miocene.
Although divergence estimates are not yet available for many groups on the oceanic islands of the Gulf of Guinea, the emerging pattern supports high species turnover. This turnover likely arises as a product of the small size of the islands, moderate distances to the continental pool of new potential dispersers, and the intense recent volcanism of the islands. Small island size is associated with high extinction rates (MacArthur and Wilson 1967). Proximity to the mainland increases colonization rates, in particular for more mobile organisms, such as birds, which is expected to favor taxon cycle dynamics. According to the taxon cycle hypothesis, successful colonizers tend to be generalists that will outcompete specialized endemics, but these generalists then evolve into endemic specialists that will themselves be outcompeted when new generalists reach the island (Wilson 1961; Ricklefs and Bermingham 1999, 2002). In the oceanic islands of the Gulf of Guinea, the community assembly of avian blood parasites is concordant with the taxon cycle hypothesis (Loiseau et al. 2017). This is particularly interesting as the co-evolutionary arms race between pathogens and their hosts has been proposed as a factor that could be driving taxon cycle patterns in the macro-fauna (Ricklefs and Bermingham 1999, 2002; Ricklefs et al. 2016). It is unclear whether such a pattern is present in other taxonomic groups in the Gulf of Guinea.
Volcanism in the continental and oceanic sector of the Cameroon Volcanic Line has been contemporaneous and more or less continuous since the Cretaceous (Fitton 1987; Lee et al. 1994; Burke 2001). Volcanic activity persisted until recently on the islands (0.1 Ma: Lee et al. 1994; 0.036 Ma: Barfod and Fitton 2014—see also Ceríaco et al. 2022c) and is still present for Mount Cameroon, and to a lesser extent, Bioko. Only faint signatures of volcanic activity remain on São Tomé in the form of hot springs (Henriques and Neto 2015), but the intensity of recent volcanic activity is still clearly visible in the orography, which is marked by high mountains and steep slopes, characteristic of young islands. For example, the peak of São Tomé, rising at 2024 m, was formed 1.5 Ma together with most of the central mountain massif of the island (Caldeira et al. 2003). These major volcanic events have likely driven species to extinction on multiple occasions, contributing to accelerating species turnover. Volcanic eruptions could also contribute to diversification and speciation, however, by dividing the ranges of previously panmictic populations with lava flows. The distributions of the two endemic sister caecilian lineages—Schistometopum ephele and S. thomense on São Tomé align with this hypothesis (Stoelting et al. 2014, O’Connell et al. 2021), as do patterns of genetic variation in the skink Trachylepis thomensis (Jesus et al. 2005).
The prevalence of species derived from recent speciation events attests to the Gulf of Guinea islands being diversification centers rather than simple “museums” where continental species found refuge from habitat changes associated with glacial cycles. Yet, the presence of species whose arrivals date to earlier times of island formation, and of Afromontane paleo-endemic plants in particular, supports the hypothesis that the islands offered a stable climatic environment during glacial cycles (Plana et al. 2004). As more dated phylogenetic studies become available from a greater diversity of taxa, we will start to gain a better understanding of the tempo of island colonization and in situ diversification.
Hybridization and Speciation in the Gulf of Guinea
The role of hybridization in evolution, and in speciation in particular, remains one of the most fundamental questions in evolutionary biology (Abbott et al. 2013; Seehausen et al. 2014; Taylor and Larson 2019). The consequences of hybridization between two lineages range from the extinction of one lineage (via the fusion of the two) to the origin of a new “hybrid” lineage. In between these extremes, hybridization can lead to different levels of genetic introgression across species boundaries, with the potential of accelerating, rather than hindering, the evolutionary process (Anderson and Stebbins 1954; Arnold and Emms 1998). In intermediate island systems, such as the oceanic islands of the Gulf of Guinea, a mainland lineage may colonize the islands more than once and at different points in time. Such cases are likely to lead to hybridization between the diverging island and mainland lineages. These rare and episodic hybridization events provide clear-cut models to study the consequences of hybridization in lineage divergence and speciation. In the oceanic islands of the Gulf of Guinea, several examples of hybridization between species have been detected. Interestingly, several of these cases were only detected with molecular data, suggesting that several other cases are likely to be uncovered with the increasing use of genetic data in the region. Here we highlight some of the better studied examples.
The Drosophila santomea x D. yakuba Hybrid Zones
The genus Drosophila, with c. 1500 species, includes the most widely used model organism for the study of genetics, D. melanogaster. Interestingly, up until the discovery and description of the endemic species from São Tomé, D. santomea (Lachaise et al. 2000), stable hybrid zones within the genus were unknown. The description of this hybrid zone in Drosophila quickly led to the search, and discovery, of others such as on the island of Bioko (Cooper et al. 2018) and the Seychelles (Matute and Ayroles 2014). On São Tomé, the island endemic co-occurs with its sister species, the widespread Sub-Saharan D. yakuba. The endemic species is mostly restricted to mist forest at higher elevations, whereas the cosmopolitan species prefers more open habitats at lower elevations. Although the two species diverged between c. 400,000 (Llopart et al. 2002) and 1 million years ago (Turissini and Matute 2017), hybridization occurs at a rate of about 1% where their ranges meet, at intermediate elevations (Lachaise et al. 2000; Llopart et al. 2005a). This hybrid zone became an important model for research on the genetic basis of phenotypic differences (Llopart et al. 2002); the evolution of reproductive barriers (Coyne et al. 2002; Moehring et al. 2006a, b; Turissini et al. 2015); the impacts of introgression on the genome, including the replacement of the mitochondrial DNA of D. santomea by that of D. yakuba (Llopart et al. 2005a; Turissini et al. 2015); and, more generally, on the role of hybridization and introgression in speciation (Turissini and Matute 2017; Matute et al. 2020). The hybrid zone is unusual in that a population of hybrid males is restricted to the higher elevations of São Tomé, away from the ranges of both parental species (Llopart et al. 2005b). The origins of this hybrid male population are still unclear.
The Hyperolius thomensis x H. molleri Hybrid Zone
Two endemic species of reed frogs occur on São Tomé—H. thomensis mostly restricted to the native closed-canopy forests, and H. molleri associated with more open habitats, including human modified ones (Bell et al. 2015b, 2022). Although closely related (c. 0.5–1.5 Ma; Bell et al. 2015a), they are clearly phenotypically distinct species differing in size, coloration, advertisement call, and reproductive biology (Drewes and Wilkinson 2004; Gilbert and Bell 2018; Bell and Irian 2019). In spite of this, hybridization occurs where the two species meet at the interface of closed-canopy forest and more open habitats, resulting in a mosaic hybrid zone (Bell et al. 2015b; Bell and Irian 2019). This hybrid zone is ripe for investigations of the genetic basis of phenotypic differences, reproductive barriers, the scale and pattern of introgression across species boundaries, and the impact of gene flow on genome architecture and phenotypic evolution. Although the geographic and temporal extent of hybridization between these species is incompletely understood, recent evidence suggests that hybridization may be a direct result of human-driven habitat changes, and of deforestation in particular (Bell and Irian 2019). The hybrid zone of the two Drosophila species from São Tomé also coincides with the transition from agricultural areas to native forest habitats, and therefore may also be a result of changes in habitat structure (Lachaise et al. 2000).
Mitochondrial Introgression in Parrots and Pigeons
Mitochondrial data used to infer phylogenetic and phylogeographic relationships of most endemic bird species in the archipelago (Melo 2007; Melo et al. 2022) uncovered several instances of mitochondrial introgression (Box 21.1 in Melo et al. 2022): (1) the Gray Parrot Psittacus erithacus, where a distinct Príncipe lineage hybridized with recent arrivals from the mainland (Melo and O'Ryan 2007); (2) the Lemon Dove Columba larvata, where, as with the parrot, a distinct island lineage was recently joined by a new wave of mainland colonizers (Hugo Pereira and Martim Melo, unpublished data); (3) from the Sao Tome Green-Pigeon Treron sanctithomae to the Príncipe subspecies of the African Green-Pigeon Treron calvus virescens (Pereira 2013). These are all species with strong flying abilities—making them typical oceanic island colonizers—and as such, secondary contact and inter-island dispersal events are not surprising. Genomic studies are required to better understand the extent of introgression derived from interbreeding between the diverging lineages.
The Saga of the Canaries Crithagra concolor x C. rufobrunnea
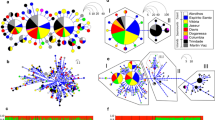
The islands of Príncipe and São Tomé host two endemic canaries (Fringillidae: Crithagra). The Principe Seedeater C. rufobrunnea is present on Príncipe, Boné de Jóquei Islet (c. 2.5 km from Príncipe), and São Tomé. Gene flow between the three allopatric populations is reduced and phenotypic differentiation has evolved, justifying their current treatment as three distinct subspecies (Melo 2007). The Sao Tome Grosbeak C. concolor is restricted to the primary forests of São Tomé, where it is the rarest or, at least, the most difficult bird species to find. The São Tomé population of the Principe Seedeater occurs across the entire island, from primary forest to urban areas—whenever trees are present. The phenotype of the grosbeak has often misled taxonomists, who episodically considered it to be a weaver (Ploceidae; cf. Melo et al. 2022). More recently, molecular evidence confirmed not only that it is a Crithagra canary, but that it is sister to the Principe Seedeater (Melo et al. 2017). The surprising twist to this story is that molecular data, from multiple loci (2 mitochondrial markers, 33 nuclear introns and exons, 34 microsatellites, and c. 10,000 single nucleotide polymorphisms—SNPs) consistently indicated that the São Tomé population of the seedeater is more closely related to the grosbeak than to its conspecific allopatric populations on Príncipe and Boné de Jóquei Islet (Melo 2007; Stervander 2009, 2015). The paraphyly of the seedeater was concordant with the grosbeak and seedeater having speciated in sympatry on São Tomé—a very unlikely scenario for birds, with the only another potential case described in the Nesospiza buntings (Thraupidae) from the Tristan da Cunha archipelago (Ryan et al. 2007). Clarification of this pattern was only possible using a large-scale genomic approach (Stervander 2015; Stervander et al. 2022). Mapping of over 130,000 SNPs across the genome revealed that the “sympatric speciation pattern” was the consequence of an extensive degree of genetic introgression between the two species. For the subset of phylogenetically informative SNPs, more SNPs supported the sympatric speciation pattern (“introgressed markers”) than allopatric speciation (i.e., where the three seedeater populations make a monophyletic group, sister to the grosbeak; “preserved markers”; Fig. 6.2). Many of the SNPs supporting allopatric speciation were associated with coding regions, including those harboring genes underlying bill size and shape, suggesting a strong role for natural selection against hybrids with intermediate bills (Stervander 2015; Stervander et al. 2022).
Genomic patterns of differentiation and introgression between the Sao Tome Grosbeak and the Principe Seedeater, exemplified by data from chromosome 14. Three classes of phylogenetic signatures are distributed across the genome: Chromosomal segments that are phylogenetically inconclusive are gray (“inconclusive”; 89.1% of all segments across the genome that were assigned a local phylogeny); Segments representing a preserved phylogenetic signal (not introgressed during secondary contact of the grosbeak and seedeater on São Tomé), where the three seedeater populations are sister taxa and form a distinct lineage from the grosbeak, are blue (“preserved” 4.6%); Segments representing introgression from the grosbeak to the São Tomé population of Príncipe seedeater are red (“introgressed”; 6.3%). The latter suggest the sympatric populations of the grosbeak and the seedeater on São Tomé are sister taxa, and divergent from the other seedeater populations. Example topologies representative of each of the three genomic classes are drawn in corresponding colors, with the three seedeater populations abbreviated as ST (São Tomé; orange font; sympatric with the grosbeak), P (Príncipe; purple), and B (Boné de Jóquei; green). Figure from Stervander et al. (2022)
Evolution on Islands
The “Island Syndrome”
Island organisms often capture the imagination of scientists and non-scientists alike, as “museums of curiosities.” They are lands of “dragons” (Komodo dragon Varanus komodoensis) and of other fantastical creatures such as the Dodo Raphus cucullatus (Hume 2012), a giant flightless pigeon encountered by Alice in her adventures in wonderland (Carroll 1865). Naturalists noticed early on that organisms on islands across the world appear to have shared suites of unusual characteristics (Darwin 1859; Wallace 1880; Carlquist 1965; Grant 1998b; Whittaker 1998). These common evolutionary paths have been described for many traits and grouped under the “island syndrome” umbrella (Grant 1998b; Losos and Ricklefs 2009; Burns 2019; Baeckens and Van Damme 2020). Such island syndrome traits include:
-
1.
increased longevity and lower fecundity (e.g., Adler and Levins 1994; Covas 2012; Novosolov et al. 2013);
-
2.
wider ecological niches (Grant 1965a, 1998b; Blondel 2000; Covas 2016; Scott et al. 2003; Amorim et al. 2017);
-
3.
small species becoming larger and large species becoming smaller (Grant 1965a; Lomolino 2005; Clegg 2010; Lomolino et al. 2013; Novosolov et al. 2013; Biddick et al. 2019; Benítez-López et al. 2021);
-
4.
species becoming more sedentary, with the evolution of flightlessness in animals (Diamond 1981; Wright et al. 2016; Leihy and Chown 2020) and the transition away from wind-dispersal in plants (Cody and Overton 1996; Kavanagh and Burns 2014);
-
5.
animals becoming less territorial, allowing them to live in higher densities (density compensation: MacArthur et al. 1972), which is also likely associated with the evolution of increased “tameness” on islands;
-
6.
birds losing colorful ornaments (Grant 1965b; Doutrelant et al. 2016).
Most of the hypotheses proposed to explain the convergent evolution of a wide suite of traits on oceanic islands are linked to the defining abiotic factors of oceanic islands: isolation, small size, and a stable and mild climate associated with the buffer influence of the sea (Grant 1998a, b; Whittaker 1998; Blondel 2000; Covas 2016; Baeckens and Van Damme 2020). Isolation and small size underlie the defining biotic feature of oceanic islands: lower species richness relative to mainland areas of equivalent size (MacArthur and Wilson 1967). This depauperate biota translates to lower levels of inter-specific competition which contributes to ecological release (Herrmann et al. 2020). Lower species richness also translates to fewer predators and parasites, allowing species to evolve in ways that are generally not possible on the mainland—including growing towards their metabolic optimum size or losing dispersal abilities.
The Gulf of Guinea islands present several potential cases of the island syndrome across different groups, albeit studies on this subject have focused primarily on birds (Box 6.2). Gigantism is the most striking, with examples found in plants, amphibians, reptiles, and birds, and dwarfism in the only endemic bird descending from a large continental species, the Sao Tome Ibis Bostrychia bocagei. Many of the island syndromes can only be identified once the island endemics are placed in the evolutionary context of the continental lineage they arose from. For instance, the endemic giant lobelia, Lobelia barnsii, found near the peak of São Tomé, is most likely part of the monophyletic clade that groups all giant Lobelia of the world (Antonelli 2008, 2009: L. barnsii not included in the analyses) and, if so, the large size of the island endemic will reflect shared history rather than convergent evolution towards island gigantism. By contrast, the Principe Giant Tree Frog Leptopelis palmatus does not appear to be closely related to the largest continental species in the genus and may therefore represent a true case of island gigantism (Jaynes et al. 2021). The house snakes, Boaedon bedriagae from São Tomé and B. mendesi from Príncipe, may also be island giants as they are considerably larger than their mainland relatives in the B. capensis complex in southern Africa (Ceríaco et al. 2021). Within an archipelago, selective pressures of the island condition may be stronger in smaller islands. This likely applies to the gigantism and tameness of the Tinhosa Grande islet population of Trachylepis adamastor. Recently described as a unique species due to its large size and dark coloration (Ceríaco 2015), molecular data indicate that the populations on Tinhosas and Príncipe are not genetically differentiated (Ceríaco et al. 2016, 2020). Such rapid phenotypic changes have been observed in just a few generations in other island lizards (e.g., Amorim et al. 2017). Likewise, out of the three populations of the endemic Principe Seedeater (São Tomé, Príncipe, Boné de Jóquei Islet), it is the birds from the small 40 ha islet that have evolved by far the largest body mass and bill and have lost more of their flying abilities (sedentariness) and anti-predator behavior (tameness) (Box 6.3).
Box 6.2 The Island Syndrome in the Birds of the Gulf of Guinea Oceanic Islands
Wider niches: Bird song is a trait directly linked to fitness for its role in mate attraction and territory defense (Collins 2004). Hence, song is a signal under strong selection for efficient transmission. In species-rich communities, competition for acoustic space is expected to be high—as overlap of different songs masks the signals and impairs the efficacy of their transmission (Wollerman and Wiley 2002). Thus, mainland species tend to partition the acoustic space into narrow temporal and spatial (frequency bandwidth) windows to minimize interference (Planqué and Slabbekoorn 2008; Weir et al. 2012). By contrast, the acoustic space of species-poor islands is predicted to be less saturated. Comparisons of bird communities of São Tomé and Cameroon revealed that the species-poor island communities live in an acoustic environment with less acoustic interference (both from birds and insects) than those on the mainland, that island species spend more time vocalizing alone, and that acoustic overlap is lower (Robert et al. 2019, 2021). This lower competition for acoustic space translates into the songs of island species occupying a broader frequency bandwidth than the songs of their mainland counterparts (Robert et al. 2021)—a pattern that is consistent with the character release hypothesis predicted from the lower levels of inter-specific competition (Grant 1972; Herrmann et al. 2020).
Island rule: The trends of body size evolution in the endemic birds of the Gulf of Guinea fit the predictions of the island rule very closely. Most small and medium birds increased in size, with three “island giants” including the world’s largest sunbird (Sao Tome Sunbird Dreptes thomensis), weaver (Giant Weaver Ploceus grandis), and canary (Sao Tome Grosbeak Crithagra rufobrunnea). The few exceptions where small birds decreased slightly in size occur in those species that co-exist with a congeneric species and, therefore, represent the few cases in which inter-specific competition is present and character displacement may be at play (see main text and Fig. 6.3). The exceptions to this rule appear to be limited to the Sao Tome Paradise-Flycatcher Terpsiphone atrochalybeia and the Sao Tome Short-tail Motacilla bocagii, which are smaller than their mainland relatives but do not have any close relatives on the island. By contrast, the only endemic derived from a group of large birds, the Sao Tome Ibis Bostrychia bocagei, is the smallest representative of its group and one of the smallest ibises in the world.
Dispersal loss: Darwin hypothesized that sedentariness on islands evolves because dispersing individuals are unlikely to return (Darwin 1859). Under this hypothesis, the smaller the island, the stronger the selection favoring individuals that do not disperse. One study investigated the evolution of flying potential among populations of the endemic Principe Seedeater Crithagra rufobrunnea in the early stages of divergence. This species occurs in three allopatric populations: Príncipe, Boné de Jóquei Islet (c. 2.5 km off Príncipe), and São Tomé. Gene flow between the three populations is very restricted and phenotypic differentiation is significant (Melo 2007). The population on the smallest island (the 40 ha Boné de Jóquei Islet) had the lowest flying potential, as inferred from its small wing length: body mass ratio (Melo 2007; Box 6.3).
Color loss: The loss of coloration, color patches, and even sexual dimorphism in island birds has long attracted the attention of ornithologists (Grant 1965b). This pattern is consistent across distinct taxonomic groups and island systems (Doutrelant et al. 2016). A trend for increased melanism has also been suggested for island birds (Uy and Vargas-Castro 2015)—and for reptiles (Novosolov et al. 2013)—but has not yet been as extensively studied. As with the island rule, color loss is on full display in the endemic birds of the oceanic islands of the Gulf of Guinea. Lipochromes (yellow and green pigments) present in mainland relatives have mostly been lost in the island endemics: Sao Tome Oriole Oriolus crassirostris, the five white-eye species (Zosterops sp.; Melo et al. 2011), the Sao Tome Sunbird, and the Principe Sunbird Anabathmis hartlaubi (Newton’s Sunbird A. newtonii being the exception). Additionally, the male of the Sao Tome Paradise-Flycatcher is entirely black, and melanin predominates in the plumage of the Principe Seedeater and the endemic São Tomé subspecies of the Western Barn Owl Tyto alba thomensis.
Several hypotheses have been put forward to explain the loss of color in island birds including that (1) species-poor communities may relax the need for elaborate signals used in species recognition (Martin et al. 2010, 2015a, b); (2) long-lived species have higher levels of parental care, which is associated with lower investment in sexual signals (Covas 2012); (3) sexual selection is relaxed as a consequence of both the reduced genetic diversity (Frankham 1997) and higher relatedness within island populations (Griffith 2000). Most work on the Gulf of Guinea islands has focused on the hypothesis that colors in birds are honest signals of immune condition as they often depend on the acquisition of carotenoids from the diet, which are also essential co-adjuvants of the immune system (Hamilton and Zuk 1982). From a pathogen perspective, islands are thought to be more benign environments because the decrease in species richness is expected to also extend to parasites. If this expectation is correct, the inter-individual variation in health condition on islands should be very narrow, and hence color would no longer hold information regarding individual condition.
A survey of avian blood parasites indicated that parasite diversity and prevalence is lower on Príncipe and São Tomé relative to the adjacent mainland (Loiseau et al. 2017). Proxies of acquired immune function were lower on islands (Lobato et al. 2017), and genes from the Major Histocompatibility Complex (also involved in acquired immunity) were found to be under relaxed selection (Barthe et al. 2022), consistent with low exposure to pathogens. Other important genetic components of the immune system, however, were impacted by small population sizes and drift rather than by relaxed selection (Barthe et al. 2022). Demonstrating a direct link between the reduction in parasites and color loss in the avian community is more challenging. Although many of the island endemics have lost coloration and many of the more recent arrivals have not, the birds of Príncipe and São Tomé do not provide enough data points along the gradient of time since colonization to conclusively support the Hamilton and Zuk hypothesis.
Box 6.3 Evolution on an Island of an Island
Boné de Jóquei Islet (1) is only about 600 x 900 m and lies c. 2.5 km off the southeast coast of Príncipe (2), from which it has been separated since the last glaciation, c. 10,000 years ago. It holds an endemic subspecies of the Principe Seedeater Crithagra rufobrunnea fradei (3), which occurs at very high densities (4). Two other subspecies occur on Príncipe and on São Tomé Islands, respectively. The birds from Boné have the smallest wing relatively to their mass, indicating the loss of dispersal ability. They have evolved a high degree of tameness, reflecting evolution in a predator-free environment. They have stouter bills, which have likely evolved due to the reliance of their diet on the resources provided by the oil palm Elaeis guineensis, which constitutes the dominant vegetation. They feed both on the pollen of the male inflorescences (5) and on the fruit (6, 7). The oil palms of Boné produce giant fruits, which appear to have no parallel worldwide—and are not found on neighboring Príncipe, where both species also co-occur. The large fruits may have co-evolved as a defense against the strong predation pressure exerted by the seedeater, or as an adaptation against dispersal. (6) Large fruits from Boné oil palms in comparison with typical fruits; 15 cm ruler shown. Photo credits: Martim Melo.

Inter-Specific Competition Accelerates Phenotypic Evolution
Low levels of inter-specific competition characterize species-poor island assemblages, resulting in high levels of intra-specific competition. Both factors likely contribute to the evolution of many traits associated with the island syndrome. Research on bird speciation in the oceanic islands of the Gulf of Guinea (Melo 2007; Melo et al. 2022), however, reveals an important role for rare cases of inter-specific competition that occur when closely related lineages meet on the same island (see also: Grant 1965c)—an event that may be not so infrequent in intermediate island systems. Molecular phylogenies have shown that in most cases the most phenotypically divergent species are those that (1) evolved in sympatry with a close relative (both evolutionarily and ecologically) and (2) represent the most recent speciation events, instead of deriving from the oldest colonization events as previously assumed (Melo et al. 2022). For example, the two most phenotypically “aberrant” white-eyes, the Principe Speirops Zosterops leucophaeus and the São Tomé Speirops Z. lugubris, are sister species derived from the most recent speciation events in the Gulf of Guinea white-eye radiation (Box 21.2 in Melo et al. 2022). Furthermore, in this radiation, when two species meet it is the newcomer that changes the most (Melo et al. 2011), in a process of asymmetrical character displacement that had been predicted by theory (Doebeli and Dieckmann 2000), and confirmed in the radiation of Darwin’s finches (Petren et al. 2005).
The evolution of true giants among the Gulf of Guinea island birds seems to have resulted from the sequential effects of character release (Herrmann et al. 2020) and character displacement (Brown and Wilson 1956; Grant 1972). The three giant birds (weaver, sunbird, canary) all evolved in sympatry with a closely related lineage (the sister lineage in the case of the canary). This suggests the following history for the evolution of gigantism in the Gulf of Guinea birds, as previously suggested by Amadon (1953): (1) a colonizer arrives to an island; (2) with no direct competitors it evolves towards a generalist diet (character release), which in birds is associated with an increase in bill size and, correspondingly, body size (Grant 1965a; Blondel 2000); (3) a related lineage colonizes the island and inter-specific competition ensues; (4) for co-existence to be possible, selection drives a reduction in competition through character displacement, (5) the larger species evolves to become even larger. Morphometrics of the five-species radiation of the white-eyes of the oceanic islands of the Gulf of Guinea is strongly suggestive of character displacement as a driver of phenotypic differentiation, although in this case the larger species evolved from the secondary arrivals (Fig. 6.3).
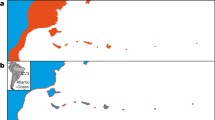
Evidence for character displacement? Morphometrics of the white-eyes (Zosteropidae) of the Gulf of Guinea, including the five-species radiation of the oceanic islands and the three species radiation of Bioko and Mount Cameroon. White-eyes generally occur as single allopatric species, but in the Gulf of Guinea there are four instances of co-occurrence of two species. On Annobón there is only one species, which has evolved a larger size than its mainland counterpart (broken line) in accordance with the island rule. In all other cases, where two species meet in sympatry, the secondary arrival (red) increased significantly in size, while the first colonizers (green) did not change much or decreased in size relative to their closest mainland relatives (depicted, approximately, by the broken line). In addition, the secondary arrivals evolved strikingly different colors from those of the typical white-eye template (Box 21.2 in Melo et al. 2022). These patterns of phenotypic divergence in this group support the process of asymmetric character displacement. The large phenotypic differences of the secondary arrivals led them to be originally placed in a separate genus, Speirops
Collectively, these studies point to the importance of inter-specific competition in driving and accelerating phenotypic divergence in island birds, and even in the speciation process.
Concluding Remarks
The Gulf of Guinea oceanic islands are an exciting example of an intermediate island system, such that they are close enough to the continent to receive a diverse array of mainland dispersers but far enough away for these to diverge once they arrive to the islands. Thus, the archipelago holds great potential for testing classic hypotheses of island biogeography by providing a wide array of independent evolutionary replicates, something that is missing from more remote archipelagos dominated by few lineages, and for investigating the role of gene flow in speciation and diversification. Likewise, the archipelago presents the opportunity to disentangle mechanisms of community assembly in a setting that is intermediate between the complex communities of continents, with high phylogenetic diversity, and the simple communities of more isolated archipelagos, in which most of the diversity is derived from a few extensive radiations. Finally, the archipelago’s endemics exhibit many of the unusual phenotypes that have long captured the attention of scientists and non-scientists, alike. As taxonomic and systematic research advances for the archipelago’s lesser known groups, hypothesis-driven studies investigating speciation and phenotypic evolution will be possible in a more representative subset of the remarkable diversity of the Gulf of Guinea oceanic islands.
References
Abbott R, Albach D, Ansell S et al (2013) Hybridization and speciation. Journal of Evolutionary Biology 26(2):229–246
Adler GH, Levins R (1994) The island syndrome in rodent populations. The Quarterly Review of Biology 69:473–490
Ali JR, Fritz U (2021) Origins of Galápagos’ land-locked vertebrates: what, whence, when, how? Biological Journal of the Linnean Society 134(2):261–284
Amadon D (1953) Avian systematics and evolution in the Gulf of Guinea. Bulletin of the American Museum of Natural History 100(3):394–451
Amorim ME, Schoener TW, Santoro GRCC, Lins ACR, Piovia-Scott J, Brandão RA (2017) Lizards on newly created islands independently and rapidly adapt in morphology and diet. Proceedings of the National Academy of Sciences 114(33):8812–8816
Anderson E, Stebbins GL (1954) Hybridization as an evolutionary stimulus. Evolution 8(4):378–388
Antonelli A (2008) Higher level phylogeny and evolutionary trends in Campanulaceae subfam. Lobelioideae: molecular signal overshadows morphology. Molecular Phylogenetics and Evolution 46(1):1–18
Antonelli A (2009) Have giant lobelias evolved several times independently? Life form shifts and historical biogeography of the cosmopolitan and highly diverse subfamily Lobelioideae (Campanulaceae). BMC Biology 7(1):82
Arnold ML (2015) Divergence with genetic exchange. Oxford University Press, Oxford
Arnold ML, Emms SK (1998) Paradigm lost: natural hybridization and evolutionary innovations. In: Howard DJ, Berlocher SH (eds) Endless forms: species and speciation. Oxford University Press, New York, pp 379–389
Baeckens S, Van Damme R (2020) The island syndrome. Current Biology 30:R338–R339
Barfod DN, Fitton JG (2014) Pleistocene volcanism on São Tomé, Gulf of Guinea, West Africa. Quaternary Geochronology 21:77–89
Barthe M, Doutrelant C, Covas R et al. (2022) Evolution of immune genes in island birds: reduction in population sizes can explain island syndrome. bioRxiv:2021.2011.2021.469450. https://doi.org/10.1101/2021.11.21.469450
Bell RC, Irian CG (2019) Phenotypic and genetic divergence in reed frogs across a mosaic hybrid zone on São Tomé island. Biological Journal of the Linnean Society 128(3):672–680
Bell RC, Drewes RC, Channing A et al (2015a) Overseas dispersal of Hyperolius reed frogs from Central Africa to the oceanic islands of São Tomé and Príncipe. Journal of Biogeography 42:65–75
Bell RC, Drewes RC, Zamudio KR (2015b) Reed frog diversification in the Gulf of Guinea: overseas dispersal, the progression rule, and in situ speciation. Evolution 69:904–915
Bell RC, Ceríaco LMP, Scheinberg LA, Drewes RC (2022) The amphibians of the Gulf of Guinea oceanic islands. In: Ceríaco LMP, Lima RF, Melo M, Bell RC (eds) Biodiversity of the Gulf of Guinea Oceanic Islands. Springer, Cham, pp 479–504
Benítez-López A, Santini L, Gallego-Zamorano J et al (2021) The island rule explains consistent patterns of body size evolution in terrestrial vertebrates. Nature Ecology and Evolution 5(6):768–786
Biddick M, Hendriks A, Burns KC (2019) Plants obey (and disobey) the island rule. Proceedings of the National Academy of Sciences 116(36):17632
Blondel J (2000) Evolution and ecology of birds on islands: trends and prospects. Vie et Milieu 50:205–220
Bolnick DI, Fitzpatrick BM (2007) Sympatric speciation: models and empirical evidence. Annual Review of Ecology, Evolution and Systematics 38:459–487
Brown WLJ, Wilson EO (1956) Character displacement. Systematic Zoology 5:49–64
Buerkle CA (2014) Gene flow, hybridization, and speciation. In: Losos JB (ed) The Princeton guide to evolution. Princeton University Press, Princeton
Burke K (2001) Origin of the Cameroon line of volcano-capped swells. Journal of Geology 109:349–362
Burns KC (2019) Evolution in isolation: the search for an island syndrome in plants. Cambridge University Press, Cambridge
Caldeira R, Madeira J, Munhá JM et al (2003) Caracterização das principais unidades vulcano-estratigráficas da ilha de São Tomé, Golfo da Guiné. Ciências da Terra (UNL) n° esp. V:A15–A18
Carlquist S (1965) Island life: a natural history of the islands of the world. The Natural History Press, New York
Carroll L (1865) Alice's adventures in wonderland. Macmillan, London
Carson HL, Clague DA (1995) Geology and biogeography of the Hawaiian islands. In: Wagner WL, Funk VA (eds) Hawaiian biogeography: evolution on a hot spot archipelago. Smithsonian Institution Press, Washington, pp 14–29
Carson HL, Kaneshiro KY (1976) Drosophila of Hawaii: systematics and ecological genetics. Annual Review of Ecology and Systematics 7:311–345
Ceríaco LMP (2015) Lost in the middle of the sea, found in the back of the shelf: a new giant species of Trachylepis (Squamata: Scincidae) from Tinhosa Grande islet, Gulf of Guinea. Zootaxa 3973(3):511–527
Ceríaco LMP, Marques MP, Bauer AM (2016) A review of the genus Trachylepis (Sauria: Scincidae) from the Gulf of Guinea, with descriptions of two new species in the Trachylepis maculilabris (Gray, 1845) species complex. Zootaxa 4109(3):284–314
Ceríaco LMP, Bernstein SAC et al (2020) The reptiles of Tinhosa Grande islet (Gulf of Guinea): a taxonomic update and the role of Quaternary Sea level fluctuations in their diversification. African Journal of Herpetology 69(2):200–2016
Ceríaco LMP, Arellano AL, Jadin R et al (2021) Taxonomic revision of the jita snakes (Lamprophiidae: Boaedon) from São Tomé and Príncipe (Gulf of Guinea), with the description of a new species. African Journal of Herpetology 70(1):1–31
Ceríaco LMP, Lima RF, Bell RC, Melo M (2022a) Biodiversity in the Gulf of Guinea oceanic islands: a synthesis. In: Ceríaco LMP, Lima RF, Melo M, Bell RC (eds) Biodiversity of the Gulf of Guinea Oceanic Islands. Springer, Cham, pp 1–12
Ceríaco LMP, Marques MP, Bell RC, Bauer AM (2022b) The terrestrial reptiles of the Gulf of Guinea oceanic islands. In: Ceríaco LMP, Lima RF, Melo M, Bell RC (eds) Biodiversity of the Gulf of Guinea Oceanic Islands. Springer, Cham, pp 505–534
Ceríaco LMP, Santos BS, Lima RF, Bell RC, Norder S, Melo M (2022c) Physical geography of the Gulf of Guinea oceanic islands. In: Ceríaco LMP, Lima RF, Melo M, Bell RC (eds) Biodiversity of the Gulf of Guinea Oceanic Islands. Springer, Cham, pp 13–36
Clegg SM (2010) Evolutionary changes following island colonization in birds: empirical insights into the roles of microevolutionary processes. In: Losos JB, Ricklefs RE (eds) The theory of island biogeography revisited. Princeton University Press, Princeton, pp 293–325
Clegg SM, Degnan SM, Kikkawa J, Moritz C, Estoup A, Owens IPF (2002) Genetic consequences of sequential founder events by an island-colonizing bird. Proceedings of the National Academy of Sciences 99:8127–8132
Cody ML, Overton JM (1996) Short-term evolution of reduced seed dispersal in island plant populations. Journal of Ecology 84:53–61
Collins S (2004) Vocal fighting and flirting: the functions of birdsong. In: Marler P, Slabbekoorn H (eds) Nature's music: the science of birdsong. Elsevier Academic Press, San Diego, pp 39–79
Cooper BS, Sedghifar A, Nash WT, Comeault AA, Matute DR (2018) A maladaptive combination of traits contributes to the maintenance of a Drosophila hybrid zone. Current Biology 28(18):2940–2947
Costa LM, Maia HA, Almeida AJ (2022) The fishes of the Gulf of Guinea oceanic islands. In: Ceríaco LMP, Lima RF, Melo M, Bell RC (eds) Biodiversity of the Gulf of Guinea Oceanic Islands. Springer, Cham, pp 431–478
Covas R (2012) Evolution of reproductive life histories in island birds worldwide. Proceedings of the Royal Society of London B 279:1531–1537
Covas R (2016) Life history evolution in island populations of birds. In: Kilman R (ed) Encyclopedia of evolutionary biology. Academic Press, Oxford, pp 352–358
Coyne JA (2007) Sympatric speciation. Current Biology 17(18):R787–R788
Coyne JA, Orr HA (2004) Speciation. Sinauer, Sunderland
Coyne JA, Kim SY, Chang AS, Lachaise D, Elwyn S (2002) Sexual isolation between two sibling species with overlapping ranges: Drosophila santomea and Drosophila yakuba. Evolution 56(12):2424–2434
Craddock EM (2000) Speciation processes in the adaptive radiation of Hawaiian plants and animals. Evolutionary Biology 31:1–53
Crews SC, Esposito LA (2022) A checklist of the arachnids from the Gulf of Guinea islands (excluding ticks and mites). In: Ceríaco LMP, Lima RF, Melo M, Bell RC (eds) Biodiversity of the Gulf of Guinea Oceanic Islands. Springer, Cham, pp 273–294
Daniel TF (2010) Sciaphila ledermannii (Triuridaceae), a biogeographically significant holosaprophyte newly reported from Príncipe in the Gulf of Guinea. Proceedings of the California Academy of Sciences 61(7):617
Darwin C (1845) In: Fitz Roy RN (ed) Journal of researches into the natural history and geology of the countries visited during the voyage of H.M.S. Beagle round the world, under the command of Capt, 2nd edn. John Murray, London
Darwin C (1859) On the origin of species by means of natural selection. John Murray, London
Desjardin DE, Perry BA (2022) Fungi of São Tomé and Príncipe Islands—basidiomycete mushrooms and allies. In: Ceríaco LMP, Lima RF, Melo M, Bell RC (eds) Biodiversity of the Gulf of Guinea Oceanic Islands. Springer, Cham, pp 189–216
Diamond JM (1981) Flightlessness and fear of flying in island species. Nature 293(5833):507–508
Dijkstra K-DB, Tate RB (2022) Dragonflies and damselflies (Odonata) of Príncipe, São Tomé and Annobón. In: Ceríaco LMP, Lima RF, Melo M, Bell RC (eds) Biodiversity of the Gulf of Guinea Oceanic Islands. Springer, Cham, pp 371–382
Doebeli M, Dieckmann U (2000) Evolutionary branching and sympatric speciation caused by different types of ecological interactions. American Naturalist 156:S77–S101
Doutrelant C, Paquet M, Renoult JP, Grégoire A, Crochet P-A, Covas R (2016) Worldwide patterns of bird colouration on islands. Ecology Letters 19(5):537–545
Dowling TE, Secor CL (1997) The role of hybridization and introgression in the diversification of animals. Annual Review of Ecology and Systematics 28:593–619
Drewes RC, Wilkinson JA (2004) The California Academy of Sciences Gulf of Guinea expedition (2001) I. The taxonomic status of the genus Nesionixalus Perret, 1976 (Anura: Hyperoliidae), treefrogs of São Tomé and Príncipe, with comments on the genus Hyperolius. Proceedings of the California Academy of Sciences 55:395–407
Emerson BC (2002) Evolution on oceanic islands: molecular phylogenetic approaches to understanding pattern and process. Molecular Ecology 11:951–966
Exell AW (1973) Angiosperms of the islands of the Gulf of Guinea (Fernando Pó, Príncipe, S. Tomé and Annobón). Bulletin of the British Museum (Natural History) Botany 4:325–411
Feder JL, Berlocher SH, Roethele JB et al (2003) Allopatric genetic origins for sympatric host-plant shifts and race formation in Rhagoletis. Proceedings of the National Academy of Sciences 100:10314–10319
Feder JL, Egan SP, Nosil P (2012) The genomics of speciation-with-gene-flow. Trends in Genetics 28(7):342–350
Figueiredo E (1994) Diversity and endemism of angiosperms in the Gulf of Guinea islands. Biodiversity and Conservation 3:785–793
Fitton JG (1987) The Cameroon line, West Africa: a comparison between oceanic and continental alkaline volcanism. In: Fitton JG, Upton BGJ (eds) Alkaline Igneous Rocks, Geological Society Special Publication No 30. Blackwell Scientific Publications, London, pp 273–291
Frankham R (1997) Do island populations have less genetic variation than mainland populations? Heredity 78:311–327
Garcia CSC, Shevock JR (2022) The bryophyte flora of São Tomé and Príncipe archipelago (Gulf of Guinea): past, present and future. In: Ceríaco LMP, Lima RF, Melo M, Bell RC (eds) Biodiversity of the Gulf of Guinea Oceanic Islands. Springer, Cham, pp 217–248
Gilbert CM, Bell RC (2018) Evolution of advertisement calls in an island radiation of African reed frogs. Biological Journal of the Linnean Society 123(1):1–11
Gillespie RG, Bennett GM, De Meester L et al. (2020) Comparing adaptive radiations across space, time, and taxa. Journal of Heredity 111(1):1–20
Grant PR (1965a) The adaptive significance of some size trends is island birds. Evolution 19:355–367
Grant PR (1965b) Plumage and the evolution of birds on islands. Systematic Zoology 14:47–52
Grant PR (1965c) Ecological compatibility of bird species on islands. American Naturalist 100(914):451–462
Grant PR (1972) Convergent and divergent character displacement. Biological Journal of the Linnean Society 4(1):39–68
Grant PR (1998a) Evolution on islands. Oxford University Press, Oxford
Grant PR (1998b) Patterns on islands and microevolution. In: Grant PR (ed) Evolution on islands. Oxford University Press, Oxford, pp 1–17
Grant PR (2000) R.C.L. Perkins and evolutionary radiations on islands. Oikos 89:195–201
Grant PR (2001) Reconstructing the evolution of birds on islands: 100 years of research. Oikos 92:385–403
Grant PR, Grant BR (2008) How and why species multiply: the radiation of Darwin's Finches. Princeton University Press, Princeton
Griffith SC (2000) High fidelity on islands: a comparative study of extrapair paternity in passerine birds. Behavioral Ecology 11(3):265–273
Hamilton WD, Zuk KM (1982) Heritable true fitness and bright birds: a role for parasites? Science 218:384–387
Harpp KS, Geist DJ (2018) The evolution of Galápagos volcanoes: an alternative perspective. Frontiers in Earth Science 6:50
Hayward A, Stone GN (2006) Comparative phylogeography across two trophic levels: the oak gall wasp Andricus kollari and its chalcid parasitoid Megastigmus stigmatizans. Molecular Ecology 15(2):479–480
Henriques MH, Neto K (2015) Geoheritage at the equator: selected geosites of São Tomé island (Cameron line, Central Africa). Sustainability 7:48–667
Herrmann NC, Stroud JT, Losos JB (2020) The evolution of 'ecological release' into the 21st century. Trends in Ecology and Evolution 36:206–215
Hume JP (2012) The dodo: from extinction to the fossil record. Geol Today 28:147–151
International Union for Conservation of Nature (2015) Ecosystem profile: Guinean forests of West Africa biodiversity hotspot. Critical Ecosystem Partnership Fund, Arlington
Jaynes KE, Myers EA, Drewes RC, Bell RC (2021) New evidence for distinctiveness of the island-endemic Príncipe giant tree frog (Arthroleptidae: Leptopelis palmatus). Herptological Journal 31:162–169
Jesus J, Harris DJ, Brehm A (2005) Phylogeography of Mabuya maculilabris (Reptilia) from São Tomé island (Gulf of Guinea) inferred from mtDNA sequences. Molecular Phylogenetics and Evolution 37:503–510
Jourdin F, Froidefond JM, Loyer S et al (2006) Measuring upper ocean turbidity off Congo and Gabon coasts. Proceedings of Caracterisation du Milieu Marin 6:16–19
Kaneshiro KY, Gillepsie RG, Carson HL (1995) Chromosomes and male genitalia of Hawaiian Drosophila: tools for interpreting phylogeny and geography. In: Wagner WL, Funk VA (eds) Hawaiian biogeography: evolution on a hot spot archipelago. Smithsonian Institution Press, Washington, pp 57–71
Kavanagh PH, Burns KC (2014) The repeated evolution of large seeds on islands. Proceedings of the Royal Society B: Biological Sciences 281(1786):20140675
Lachaise D, Harry M, Solignac M, Lemeunier F, Bénassi V, Cariou ML (2000) Evolutionary novelties in islands: Drosophila santomea, a new melanogaster sister species from São Tomé. Proceedings of the Royal Society of London Series B: Biological Sciences 267(1452):1487–1495
Lee D-C, Halliday AN, Fitton JG, Poli G (1994) Isotopic variations with distance and time in the volcanic islands of the Cameroon line: evidence for a mantle plume origin. Earth and Planetary Science Letters 123:119–138
Leihy RI, Chown SL (2020) Wind plays a major but not exclusive role in the prevalence of insect flight loss on remote islands. Proceedings of the Royal Society B: Biological Sciences 287(1940):20202121
Lézine A-M, Tastet J-P, Leroux M (1994) Evidence of atmospheric paleocirculation over the Gulf of Guinea since the last glacial maximum. Quaternary Research 41:390–395
Llopart A, Elwyn S, Lachaise D, Coyne AJ (2002) Genetics of a difference in pigmentation between Drosophila yakuba and Drosophila santomea. Evolution 56(11):2262–2277
Llopart A, Lachaise D, Coyne JA (2005a) Multilocus analysis of introgression between two sympatric sister species of drosophila: Drosophila yakuba and D. santomea. Genetics 171(1):197
Llopart A, Lachaise D, Coyne AJ (2005b) An anomalous hybrid zone in Drosophila. Evolution 59(12):2602–2607
Loader SP, Pisani D, Cotton JA, Gower DJ, Day JJ, Wilkinson M (2007) Relative time scales reveal multiple origins of parallel disjunct distributions of African caecilian amphibians. Biology Letters 3(5):505–508
Lobato E, Doutrelant C, Melo M, Reis S, Covas R (2017) Insularity effects on bird immune parameters: a comparison between island and mainland populations in West Africa. Ecology and Evolution 7:3645–3656
Loiseau C, Melo M, Lobato E et al (2017) Insularity effects on the assemblage of the blood parasite community of the birds from the Gulf of Guinea. Journal of Biogeography 44(11):2607–2617
Loiseau C, Melo M, Lee Y et al (2019) High endemism of mosquitoes on São Tomé and Príncipe Islands: evaluating the general dynamic model in a worldwide island comparison. Insect Conservation and Diversity 12:69–79
Lomolino MV (2005) Body size evolution in insular vertebrates: generality of the island rule. Journal of Biogeography 32(10):1683–1699
Lomolino MV, Geer AA, Lyras GA, Palombo MR, Sax DF, Rozzi R (2013) Of mice and mammoths: generality and antiquity of the island rule. Journal of Biogeography 40(8):1427–1439
Losos JB, Ricklefs RE (2009) Adaptation and diversification on islands. Nature 457:830–836
MacArthur RH, Wilson EO (1967) The theory of island biogeography. Princeton University Press, Princeton
MacArthur RH, Diamond JM, Karr JR (1972) Density compensation in island faunas. Ecology 53:330–342
Martin PR, Montgomerie R, Lougheed SC (2010) Rapid sympatry explains greater color pattern divergence in high latitude birds. Evolution 64(2):336–347
Martin CH, Cutler JS, Friel JP, Touokong CD, Coop G, Wainwright PC (2015a) Complex histories of repeated gene flow in Cameroon crater lake cichlids cast doubt on one of the clearest examples of sympatric speciation. Evolution 69:1406–1422
Martin PR, Montgomerie R, Lougheed SC (2015b) Color patterns of closely related bird species are more divergent at intermediate levels of breeding-range sympatry. The American Naturalist 185(4):443–451
Matute DR, Ayroles JF (2014) Hybridization occurs between Drosophila simulans and D. sechellia in the Seychelles archipelago. Journal of Evolutionary Biology 27:1057–1068
Matute DR, Cooper BS (2021) Comparative studies on speciation: 30 years since Coyne and Orr. Evolution 75(4):764–778
Matute DR, Comeault AA, Earley E et al (2020) Rapid and predictable evolution of admixed populations between two Drosophila species pairs. Genetics 214(1):211
Mayr E, Diamond JM (2001) The birds of northern Melanesia: speciation, ecology, and biogeography. Oxford University Press, Oxford
Measey GJ, Vences M, Drewes RC, Chiari Y, Melo M, Bourles B (2007) Freshwater paths into the ocean: molecular phylogeny of the frog Ptychadena newtoni gives insights into amphibian colonisation of oceanic islands. Journal of Biogeography 34:7–20
Melo M (2007) Bird speciation in the Gulf of Guinea. PhD thesis. University of Edinburgh, Edinburgh
Melo M, O'Ryan C (2007) Genetic differentiation between Príncipe island and mainland populations of the grey parrot (Psittacus erithacus), and implications for conservation. Molecular Ecology 16:1673–1685
Melo M, Warren BH, Jones PJ (2011) Rapid parallel evolution of aberrant traits in the diversification of the Gulf of Guinea white-eyes (Aves, Zosteropidae). Molecular Ecology 20:4953–4967
Melo M, Stervander M, Hansson B, Jones PJ (2017) The endangered São Tomé grosbeak Neospiza concolor is the world's largest canary. Ibis 159:673–679
Melo M, Jones PJ, Lima RF (2022) The avifauna of the Gulf of Guinea oceanic islands. In: Ceríaco LMP, Lima RF, Melo M, Bell RC (eds) Biodiversity of the Gulf of Guinea Oceanic Islands. Springer, Cham, pp 555–592
Mendes LF, Bivar-de-Sousa A (2022) Butterflies and skippers (Lepidoptera: Papilionoidea) of the Gulf of Guinea oceanic islands. An updated checklist. In: Ceríaco LMP, Lima RF, Melo M, Bell RC (eds) Biodiversity of the Gulf of Guinea Oceanic Islands. Springer, Cham, pp 349–370
Miller EC, Sellas AB, Drewes RC (2012) A new species of Hemidactylus (Squamata: Gekkonidae) from Príncipe island, Gulf of Guinea, West Africa with comments on the African–Atlantic clade of Hemidactylus geckos. African Journal of Herpetology 61(1):40–57
Moehring AJ, Llopart A, Elwyn S, Coyne JA, Mackay TFC (2006a) The genetic basis of prezygotic reproductive isolation between Drosophila santomea and D. yakuba due to mating preference. Genetics 173(1):215–223
Moehring AJ, Llopart A, Elwyn S, Coyne JA, Mackay TFC (2006b) The genetic basis of postzygotic reproductive isolation between Drosophila santomea and D. yakuba due to hybrid male sterility. Genetics 173(1):225–233
Mustapha M, McCall PJ, Cheke RA, Post RJ (2006) The blackflies (Diptera: Simuliidae) of Bioko (Republic of Equatorial Guinea) and the Gulf of Guinea with a description of the larvae of the ‘Pomeroy’ form of Simulium cervicornutum. Systematic Entomology 31:611–620
Nève G, Bonneau P, Coache A, Serrano A, Filippi G (2022) The beetles (Coleoptera) of Príncipe, São Tomé and Annobón. In: Ceríaco LMP, Lima RF, Melo M, Bell RC (eds) Biodiversity of the Gulf of Guinea Oceanic Islands. Springer, Cham, pp 295–348
Nicolas V, Jacquet F, Hutterer R et al (2019) Multilocus phylogeny of the Crocidura poensis species complex (Mammalia, Eulipotyphla): influences of the palaeoclimate on its diversification and evolution. Journal of Biogeography 46(5):871–873
Nosil P (2012) Ecological speciation. In: Oxford series in ecology and evolution. Oxford University Press, Oxford
Novosolov M, Raia P, Meiri S (2013) The island syndrome in lizards. Global Ecology and Biogeography 22:184–191
O’Connell KA, Prates I, Scheinberg LA, Mulder KP, Bell RC (2021) Speciation and secondary contact in a fossorial island endemic, the São Tomé caecilian. Molecular Ecology 30:2859–2871
Panisi M, Lima RF, Lima JC et al (2022) Terrestrial mollusca of the Gulf of Guinea oceanic islands. In: Ceríaco LMP, Lima RF, Melo M, Bell RC (eds) Biodiversity of the Gulf of Guinea Oceanic Islands. Springer, Cham
Pereira H (2013) Conservation genetics of the endemic pigeons of São Tomé and Príncipe. MSc thesis. Universidade do Porto, Porto
Perkins RCL (1901) An introduction to the study of the Drepanidae, a family of birds peculiar to the Hawaiian islands. Ibis 1(8):562–585
Perkins RCL (1903) Vertebrata. In: Sharp D (ed) Fauna Hawaiiensis. Cambridge University Press, Cambridge, pp 365–466
Perkins RCL (1913) Introduction. In: Sharp D (ed) Fauna Hawaiiensis. Cambridge University Press, Cambridge, pp xv–ccxxviii
Petren K, Grant PR, Grant BR, Keller LF (2005) Comparative landscape genetics and the adaptive radiation of Darwin's finches: the role of peripheral isolation. Molecular Ecology 14:2943–2957
Pinho C, Hey J (2010) Divergence with gene flow: models and data. Annual Review of Ecology, Evolution and Systematics 41:215–230
Plana V, Gascoigne A, Forrest LL, Harris D, Pennington RT (2004) Pleistocene and pre-Pleistocene Begonia speciation in Africa. Molecular Phylogenetics and Evolution 31:449–461
Planqué R, Slabbekoorn H (2008) Spectral overlap in songs and temporal avoidance in a Peruvian bird assemblage. Ethology 114(3):262–271
Rainho A, Meyer CFJ, Thorsteinsdóttir S, Juste J, Palmeirim JM (2022) Current knowledge and conservation of the wild mammals of the Gulf of Guinea oceanic islands. In: Ceríaco LMP, Lima RF, Melo M, Bell RC (eds) Biodiversity of the Gulf of Guinea Oceanic Islands. Springer, Cham, pp 593–620
Richardson PL, Walsh D (1986) Mapping climatological seasonal variations of surface currents in the tropical Atlantic using ship drifts. Journal of Geophysical Research 91:10537–10550
Ricklefs RE, Bermingham E (1999) Taxon cycles in the lesser Antillean avifauna. Ostrich 70:49–59
Ricklefs RE, Bermingham E (2002) The concept of the taxon cycle in biogeography. Global Ecology and Biogeography 11:353–361
Ricklefs RE, Bermingham E (2007) The causes of evolutionary radiations in archipelagoes: passerine birds in the Lesser Antilles. American Naturalist 169:285–297
Ricklefs RE, Soares L, Ellis VA, Latta SC (2016) Haemosporidian parasites and avian host population abundance in the Lesser Antilles. Journal of Biogeography 43:1277–1286
Robert A, Lengagne T, Melo M et al (2019) The theory of island biogeography and soundscapes: species diversity and the organization of acoustic communities. Journal of Biogeography 46:1901–1911
Robert A, Melo M, Lengagne T, Julien S, Gomez D, Doutrelant C (2021) Patterns of bird song evolution on islands support the character release hypothesis in tropical but not in temperate latitudes. Journal of Evolutionary Biology 34:1580–1591
Rundell RJ, Price TD (2009) Adaptive radiation, nonadaptive radiation, ecological speciation and nonecological speciation. Trends in Ecology and Evolution 24:394–399
Rundle HD, Nosil P (2005) Ecological speciation. Ecology Letters 8:336–352
Ryan PG, Bloomer P, Moloney CL, Grant TJ, Delport W (2007) Ecological speciation in South Atlantic island finches. Science 315:1420–1423
Schalansky J (2010) Atlas of remote islands: fifty islands I have never visited and never will. Penguin Books, London
Schluter D (2000) The ecology of adaptive radiation. Oxford University Press, Oxford
Scott SN, Clegg SM, Blomberg SP, Kikkawa J, Ian PFO (2003) Morphological shifts in island-dwelling birds: the roles of generalist foraging and niche expansion. Evolution 57(9):2147–2156
Seehausen O (2004) Hybridization and adaptive radiation. Trends in Ecology and Evolution 19(4):198–207
Seehausen O, Butlin RK, Keller I et al (2014) Genomics and the origin of species. Nature Reviews Genetics 15(3):176–192
Stervander M (2009) Tracking genetic divergence in Gulf of Guinea finches - strong support for sympatric speciation. MSc Thesis. Lund University, Lund
Stervander M (2015) On speciation in birds—genomic signatures across space and time. PhD thesis. University of Lund, Lund
Stervander M, Melo M, Jones PJ, Hansson B (2022) Genomic signatures of isolation, hybridization, and selection during speciation of island finches. bioRxiv:2022.2001.2005.474904. https://doi.org/10.1101/2022.01.05.474904
Stévart T, Dauby G, Ikabanga DU et al (2022) Diversity of the vascular plants of the Gulf of Guinea oceanic islands. In: Ceríaco LMP, Lima RF, Melo M, Bell RC (eds) Biodiversity of the Gulf of Guinea Oceanic Islands. Springer, Cham, pp 249–272
Stoelting RE, Measey GJ, Drewes RC (2014) Population genetics of the São Tomé Caecilian (Gymnophiona: Dermophiidae: Schistometopum thomense) reveals strong geographic structuring. PLoS One 9(8):e104628
Taylor SA, Larson EL (2019) Insights from genomes into the evolutionary importance and prevalence of hybridization in nature. Nature Ecology and Evolution 3(2):170–177
Turissini DA, Matute DR (2017) Fine scale mapping of genomic introgressions within the Drosophila yakuba clade. PLoS Genetics 13(9):e1006971
Turissini DA, Liu G, David JR, Matute DR (2015) The evolution of reproductive isolation in the Drosophila yakuba complex of species. Journal of Evolutionary Biology 28:557–575
Uy JAC, Vargas-Castro LE (2015) Island size predicts the frequency of melanic birds in the color-polymorphic flycatcher Monarcha castaneiventris of the Solomon Islands. The Auk 132(4):787–794
Valente L, Phillimore AB, Melo M et al (2020) A simple dynamic model explains the diversity of island birds worldwide. Nature 579(7797):92–96
Vences M (2005) Madagascar as a model region for the study of tempo and pattern in adaptive radiations. In: Huber BA, Sinclair BJ, Lampe K-H (eds) African biodiversity: molecules, organisms, ecosystems. Springer, New York
Wagner WL, Funk VA (eds) (1995) Hawaiian biogeography: evolution on a hot spot archipelago. Smithsonian Institution Press, Washington
Wallace AR (1880) Island life: or, the phenomena and causes of insular faunas and floras, including a revision and attempted solution of the problem of geological climates. Macmillan, London
Weigelt P, Kreft H (2013) Quantifying island isolation—insights from global patterns of insular plant species richness. Ecography 36:417–419
Weinell JL, Branch WR, Colston TJ et al (2019) A species-level phylogeny of Trachylepis (Scincidae: Mabuyinae) provides insight into their reproductive mode evolution. Molecular Phylogenetics and Evolution 136:183–195
Weir JT, Wheatcroft DJ, Price TD (2012) The role of ecological constraint in driving the evolution of avian song frequency across a latitudinal gradient. Evolution 66(9):2773–2783
Whittaker RJ (1998) Island biogeography: ecology, evolution, and conservation. Oxford University Press, Oxford
Whittaker RJ, Fernández-Palacios JM, Matthews TJ, Borregaard MK, Triantis KA (2017) Island biogeography: taking the long view of nature’s laboratories. Science 357(6354):eaam8326
Wilson EO (1961) The nature of the taxon cycle in the Melanesian ant fauna. The American Naturalist 95(882):169–193
Wollerman L, Wiley HR (2002) Possibilities for error during communication by neotropical frogs in a complex acoustic environment. Behavioral Ecology and Sociobiology 52(6):465–473
Wright NA, Steadman DW, Witt CC (2016) Predictable evolution toward flightlessness in volant island birds. Proceedings of the National Academy of Sciences 113(17):4765
Zimkus BM, Lawson LP, Barej MF et al (2017) Leapfrogging into new territory: how Mascarene ridged frogs diversified across Africa and Madagascar to maintain their ecological niche. Molecular Phylogenetics and Evolution 106:254–269
Acknowledgments
MM was supported via the European Union’s Horizon 2020 research and innovation programme under grant agreement 854248. Fundação para a Ciência e a Tecnologia (FCT, Portugal) provided structural funding to CIBIO (UIDB/50027/2021). We thank the editor Ricardo F. de Lima for his helpful comments and suggestions.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2022 The Author(s)
About this chapter
Cite this chapter
Melo, M., Ceríaco, L.M.P., Bell, R.C. (2022). Biogeography and Evolution in the Oceanic Islands of the Gulf of Guinea. In: Ceríaco, L.M.P., de Lima, R.F., Melo, M., Bell, R.C. (eds) Biodiversity of the Gulf of Guinea Oceanic Islands. Springer, Cham. https://doi.org/10.1007/978-3-031-06153-0_6
Download citation
DOI: https://doi.org/10.1007/978-3-031-06153-0_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-06152-3
Online ISBN: 978-3-031-06153-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)