Abstract
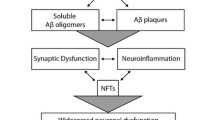
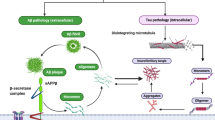
Alzheimer’s disease (AD) is the most common type of dementia caused by severe neurodegeneration in the hippocampus and neocortical regions of the brain. In addition to neurodegeneration, AD brains contain high levels of amyloid plaques (APs) and neurofibrillary tangles (NFTs) which are used as neuropathological hallmarks of the disorder. Despite intense research efforts, the mechanism(s) of the AD neurodegeneration are imperfectly understood, hampering efforts for the development of efficient therapeutics. Furthermore, failure of clinical trials to benefit AD patients suggests that AD hallmarks are poor therapeutic targets and supports the suggestion that these hallmarks are sequelae of neurodegeneration. Although genetic evidence seem to support the amyloid theory of AD, additional empirical observations and experimental data are inconsistent with the amyloid/Aβ theories of AD [Robakis and Neve (1998), TINS vol. 21 pp.15–19; Robakis (2011) NBA vol. 32, pp 372–379]. This possibility is further supported by data that amyloid plaques and neurofibrillary tangles are found in a number of distinct neurodegenerative disorders and that animal models expressing high levels of AD pathological structures show little neuronal loss. Furthermore, genetic evidence linking genetic loci to disease reveal little about the molecular mechanisms involved. Mutants of APP, PS1, and PS2 cause familial AD (FAD) suggesting these mutants can be used as models to study mechanisms of neurodegeneration. Recent reports show that the ability of efnB1 and BDNF (factors) to rescue neurons from excitotoxicity depends on PS1 but is independent of γ-secretase. Interestingly, PS1 FAD mutations block the ability of factors to protect neurons from toxicity suggesting that FAD mutants may increase neuronal death by blocking neuroprotective activities of brain neurotrophins. Other reports also suggest that proteins involved in FAD have Aβ-/γ-secretase-independent functions that can play important roles in AD. Furthermore, non-neuronal brain cells like microglia are implicated in AD pathology.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
Alzheimer’s disease (AD) is the most common type of dementia caused by severe neurodegeneration in the hippocampus and neocortical regions of the brain. In addition to neurodegeneration, AD brains contain high levels of amyloid plaques (APs) and neurofibrillary tangles (NFTs) which are used as neuropathological hallmarks of the disorder. Despite intense research efforts, the mechanism(s) of the AD neurodegeneration are imperfectly understood, hampering efforts for the development of efficient therapeutics. Furthermore, failure of clinical trials to benefit AD patients suggests that AD hallmarks are poor therapeutic targets and supports the suggestion that these hallmarks are sequelae of neurodegeneration. Although genetic evidence seem to support the amyloid theory of AD, additional empirical observations and experimental data are inconsistent with the amyloid/Aβ theories of AD [Robakis and Neve (1998), TINS vol. 21 pp.15–19; Robakis (2011) NBA vol. 32, pp 372–379]. This possibility is further supported by data that amyloid plaques and neurofibrillary tangles are found in a number of distinct neurodegenerative disorders and that animal models expressing high levels of AD pathological structures show little neuronal loss. Furthermore, genetic evidence linking genetic loci to disease reveal little about the molecular mechanisms involved. Mutants of APP, PS1, and PS2 cause familial AD (FAD) suggesting these mutants can be used as models to study mechanisms of neurodegeneration. Recent reports show that the ability of efnB1 and BDNF (factors) to rescue neurons from excitotoxicity depends on PS1 but is independent of γ-secretase. Interestingly, PS1 FAD mutations block the ability of factors to protect neurons from toxicity suggesting that FAD mutants may increase neuronal death by blocking neuroprotective activities of brain neurotrophins. Other reports also suggest that proteins involved in FAD have Aβ-/γ-secretase-independent functions that can play important roles in AD. Furthermore, non-neuronal brain cells like microglia are implicated in AD pathology.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this paper
Cite this paper
Robakis, N.K. (2020). What Do Recent Clinical Trials Teach Us About the Etiology of AD. In: Vlamos, P. (eds) GeNeDis 2018. Advances in Experimental Medicine and Biology, vol 1195. Springer, Cham. https://doi.org/10.1007/978-3-030-32633-3_23
Download citation
DOI: https://doi.org/10.1007/978-3-030-32633-3_23
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-32632-6
Online ISBN: 978-3-030-32633-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)