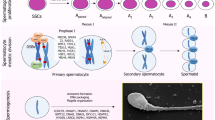
Abstract
Early in embryogenesis, cells that are destined to become germ cells take on a different destiny from other cells in the embryo. The germ cells are not programmed to perform “vital” functions but to perpetuate the species through the transfer of genetic materials to the next generation. To fulfill their destiny, male germ cells undergo meiosis and extensive morphogenesis that transforms the round-shaped cells into freely motile sperm propelled by a beating flagellum to seek out their missing half. Apparently, extra genes and additional regulatory mechanisms are required to achieve all these unique features, and an estimated 11 % of genes are involved in fertility in Drosophila (Hackstein et al., Trends Genet 16(12):565–572, 2000). If comparative numbers of male fertility genes are needed in mammals, extra risks of male fertility problems are associated with disruptive mutations in those genes. Among human male infertility cases, approximately 22 % were classified as “idiopathic,” a term used to describe diseases of unknown causes, with idiopathic oligozoospermia being the most common semen abnormality (11.2 %) (Comhaire et al., Int J Androl (Suppl 7):1–53, 1987). “Idiopathic” is a widely used adjective that is used to reflect our lack of understanding of the genetics of male fertility. Fortunately, after more than two decades of phenotypic studies using knockout mice and identifying genes disrupted in spontaneous mutant mice, we have unveiled new and unexpected aspects of crucial gene functions for fertility. Other efforts to categorize genes involved in male fertility in mammals have suggested a total of 1,188 genes (Hermo et al., Microsc Res Tech 73(4):241–494, 2010). Although intracytoplasmic sperm injection (ICSI) can be used to bypass many fertilization obstacles to achieve fertilization with only a few extracted sperm, the widespread use of ICSI without proper knowledge for genetic testing and counseling could still potentially propagate pleiotropic gene mutations associated with male infertility and other genetic diseases (Alukal and Lamb, Urol Clin North Am 35(2):277–288, 2008). In this chapter, we give a brief account of major events during the development of male germ cells and focus on the functions of several crucial genes that have been studied in mutant mouse models and are potential causes of human male infertility.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Edson MA, Nagaraja AK, Matzuk MM (2009) The mammalian ovary from genesis to revelation. Endocr Rev 30(6):624–712
Lawson KA et al (1999) Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes Dev 13(4):424–436
Ying Y et al (2000) Requirement of Bmp8b for the generation of primordial germ cells in the mouse. Mol Endocrinol 14(7):1053–1063
Chang H, Matzuk MM (2001) Smad5 is required for mouse primordial germ cell development. Mech Dev 104(1–2):61–67
Tremblay KD, Dunn NR, Robertson EJ (2001) Mouse embryos lacking Smad1 signals display defects in extra-embryonic tissues and germ cell formation. Development 128(18):3609–3621
Richardson BE, Lehmann R (2010) Mechanisms guiding primordial germ cell migration: strategies from different organisms. Nat Rev Mol Cell Biol 11(1):37–49
Manova K et al (1990) Gonadal expression of c-kit encoded at the W locus of the mouse. Development 110(4):1057–1069
Matsui Y, Zsebo KM, Hogan BL (1990) Embryonic expression of a haematopoietic growth factor encoded by the Sl locus and the ligand for c-kit. Nature 347(6294):667–669
Gu Y et al (2009) Steel factor controls primordial germ cell survival and motility from the time of their specification in the allantois, and provides a continuous niche throughout their migration. Development 136(8):1295–1303
Ohinata Y et al (2005) Blimp1 is a critical determinant of the germ cell lineage in mice. Nature 436(7048):207–213
Kurimoto K et al (2008) Specification of the germ cell lineage in mice: a process orchestrated by the PR-domain proteins, Blimp1 and Prdm14. Cell Cycle 7(22):3514–3518
Yamaji M et al (2008) Critical function of Prdm14 for the establishment of the germ cell lineage in mice. Nat Genet 40(8):1016–1022
Sakurai T et al (1995) The ter mutation first causes primordial germ cell deficiency in ter/ter mouse embryos at 8 days of gestation. Dev Growth Differ 37(3):293–302
Youngren KK et al (2005) The Ter mutation in the dead end gene causes germ cell loss and testicular germ cell tumours. Nature 435(7040):360–364
Harley VR, Clarkson MJ, Argentaro A (2003) The molecular action and regulation of the testis-determining factors, SRY (sex-determining region on the Y chromosome) and SOX9 [SRY-related high-mobility group (HMG) box 9]. Endocr Rev 24(4):466–487
Foster JW et al (1994) Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature 372(6506):525–530
Wagner T et al (1994) Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell 79(6):1111–1120
Huang B et al (1999) Autosomal XX sex reversal caused by duplication of SOX9. Am J Med Genet 87(4):349–353
Cox JJ et al (2011) A SOX9 duplication and familial 46, XX developmental testicular disorder. N Engl J Med 364(1):91–93
Vetro A et al (2011) XX males SRY negative: a confirmed cause of infertility. J Med Genet 48(10):710–712
Barsoum I, Yao HH (2006) The road to maleness: from testis to Wolffian duct. Trends Endocrinol Metab 17(6):223–228
Capel B (2006) R-spondin1 tips the balance in sex determination. Nat Genet 38(11):1233–1234
Collignon J et al (1996) A comparison of the properties of Sox-3 with Sry and two related genes, Sox-1 and Sox-2. Development 122(2):509–520
Sutton E et al (2011) Identification of SOX3 as an XX male sex reversal gene in mice and humans. J Clin Invest 121(1):328–341
d’Anglemont de Tassigny X et al (2007) Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc Natl Acad Sci U S A 104(25):10714–10719
Lapatto R et al (2007) Kiss1−/− mice exhibit more variable hypogonadism than Gpr54−/− mice. Endocrinology 148(10):4927–4936
Han SK et al (2005) Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci 25(49):11349–11356
Pineda R et al (2010) Physiological roles of the kisspeptin/GPR54 system in the neuroendocrine control of reproduction. Prog Brain Res 181:55–77
Nakagawa T et al (2010) Functional hierarchy and reversibility within the murine spermatogenic stem cell compartment. Science 328(5974):62–67
Mauduit C, Hamamah S, Benahmed M (1999) Stem cell factor/c-kit system in spermatogenesis. Hum Reprod Update 5(5):535–545
Yoshinaga K et al (1991) Role of c-kit in mouse spermatogenesis: identification of spermatogonia as a specific site of c-kit expression and function. Development 113(2):689–699
Handel MA, Schimenti JC (2010) Genetics of mammalian meiosis: regulation, dynamics and impact on fertility. Nat Rev Genet 11(2):124–136
Yang F et al (2008) Meiotic failure in male mice lacking an X-linked factor. Genes Dev 22(5):682–691
Yang F et al (2008) Mouse TEX15 is essential for DNA double-strand break repair and chromosomal synapsis during male meiosis. J Cell Biol 180(4):673–679
Berthet C et al (2003) Cdk2 knockout mice are viable. Curr Biol 13(20):1775–1785
Viera A et al (2009) CDK2 is required for proper homologous pairing, recombination and sex-body formation during male mouse meiosis. J Cell Sci 122(Pt 12):2149–2159
Yang F et al (2006) Mouse SYCP2 is required for synaptonemal complex assembly and chromosomal synapsis during male meiosis. J Cell Biol 173(4):497–507
Yuan L et al (2000) The murine SCP3 gene is required for synaptonemal complex assembly, chromosome synapsis, and male fertility. Mol Cell 5(1):73–83
Hunt PA, Hassold TJ (2002) Sex matters in meiosis. Science 296(5576):2181–2183
World Health Organization (1999) WHO Laboratory manual for the examination human semen and sperm-cervical mucus interaction. Cambridge University Press, Cambridge
Kierszenbaum AL, Tres LL (2004) The acrosome-acroplaxome-manchette complex and the shaping of the spermatid head. Arch Histol Cytol 67(4):271–284
Kang-Decker N et al (2001) Lack of acrosome formation in Hrb-deficient mice. Science 294(5546):1531–1533
Yao R et al (2002) Lack of acrosome formation in mice lacking a Golgi protein, GOPC. Proc Natl Acad Sci U S A 99(17):11211–11216
Xu X et al (1999) Globozoospermia in mice lacking the casein kinase II alpha’ catalytic subunit. Nat Genet 23(1):118–121
Lin YN et al (2007) Loss of zona pellucida binding proteins in the acrosomal matrix disrupts acrosome biogenesis and sperm morphogenesis. Mol Cell Biol 27(19):6794–6805
Xiao N et al (2009) PICK1 deficiency causes male infertility in mice by disrupting acrosome formation. J Clin Invest 119(4):802–812
Meistrich ML, Trostle-Weige PK, Russell LD (1990) Abnormal manchette development in spermatids of azh/azh mutant mice. Am J Anat 188(1):74–86
Mendoza-Lujambio I et al (2002) The Hook1 gene is non-functional in the abnormal spermatozoon head shape (azh) mutant mouse. Hum Mol Genet 11(14):1647–1658
Zhou J et al (2009) RIM-BP3 is a manchette-associated protein essential for spermiogenesis. Development 136(3):373–382
Xiao N et al (2009) PICK1 deficiency causes male infertility in mice by disrupting acrosome formation. J Clin Invest 119(4):802
Audouard C, Christians E (2011) Hsp90beta1 knockout targeted to male germline: a mouse model for globozoospermia. Fertil steril 95(4):1475–7 e1-4
Pazour GJ et al (2005) Proteomic analysis of a eukaryotic cilium. J Cell Biol 170(1):103–113
Neesen J et al (2001) Disruption of an inner arm dynein heavy chain gene results in asthenozoospermia and reduced ciliary beat frequency. Hum Mol Genet 10(11):1117–1128
Rashid S et al (2010) Disruption of the murine dynein light chain gene Tcte3-3 results in asthenozoospermia. Reproduction 139(1):99–111
Tanaka H et al (2004) Mice deficient in the axonemal protein Tektin-t exhibit male infertility and immotile-cilium syndrome due to impaired inner arm dynein function. Mol Cell Biol 24(18):7958–7964
Sapiro R et al (2002) Male infertility, impaired sperm motility, and hydrocephalus in mice deficient in sperm-associated antigen 6. Mol Cell Biol 22(17):6298–6305
Lee L et al (2008) Primary ciliary dyskinesia in mice lacking the novel ciliary protein Pcdp1. Mol Cell Biol 28(3):949–957
Schneider M et al (2009) Mitochondrial glutathione peroxidase 4 disruption causes male infertility. FASEB J 23(9):3233–3242
Imai H et al (2009) Depletion of selenoprotein GPx4 in spermatocytes causes male infertility in mice. J Biol Chem 284(47):32522–32532
Miki K et al (2004) Glyceraldehyde 3-phosphate dehydrogenase-S, a sperm-specific glycolytic enzyme, is required for sperm motility and male fertility. Proc Natl Acad Sci U S A 101(47):16501–16506
Odet F et al (2008) Expression of the gene for mouse lactate dehydrogenase C (Ldhc) is required for male fertility. Biol Reprod 79(1):26–34
Danshina PV et al (2010) Phosphoglycerate kinase 2 (PGK2) is essential for sperm function and male fertility in mice. Biol Reprod 82(1):136–145
Miki K et al (2002) Targeted disruption of the Akap4 gene causes defects in sperm flagellum and motility. Dev Biol 248(2):331–342
Ishikawa H, Kubo A, Tsukita S (2005) Odf2-deficient mother centrioles lack distal/subdistal appendages and the ability to generate primary cilia. Nat Cell Biol 7(5):517–524
Salmon NA, Reijo Pera RA, Xu EY (2006) A gene trap knockout of the abundant sperm tail protein, outer dense fiber 2, results in preimplantation lethality. Genesis 44(11):515–522
Zheng H et al (2007) Lack of Spem1 causes aberrant cytoplasm removal, sperm deformation, and male infertility. Proc Natl Acad Sci U S A 104(16):6852–6857
Bailey JL (2010) Factors regulating sperm capacitation. Syst Biol Reprod Med 56(5):334–348
Visconti PE (2009) Understanding the molecular basis of sperm capacitation through kinase design. Proc Natl Acad Sci U S A 106(3):667–668
Esposito G et al (2004) Mice deficient for soluble adenylyl cyclase are infertile because of a severe sperm-motility defect. Proc Natl Acad Sci U S A 101(9):2993–2998
Hess KC et al (2005) The “soluble” adenylyl cyclase in sperm mediates multiple signaling events required for fertilization. Dev Cell 9(2):249–259
Santi CM et al (2010) The SLO3 sperm-specific potassium channel plays a vital role in male fertility. FEBS Lett 584(5):1041–1046
Zeng XH et al (2011) Deletion of the Slo3 gene abolishes alkalization-activated K+ current in mouse spermatozoa. Proc Natl Acad Sci U S A 108(14):5879–5884
Carlson AE et al (2005) Identical phenotypes of CatSper1 and CatSper2 null sperm. J Biol Chem 280(37):32238–32244
Carlson AE et al (2003) CatSper1 required for evoked Ca2+ entry and control of flagellar function in sperm. Proc Natl Acad Sci U S A 100(25):14864–14868
Jin J et al (2007) Catsper3 and Catsper4 are essential for sperm hyperactivated motility and male fertility in the mouse. Biol Reprod 77(1):37–44
Qi H et al (2007) All four CatSper ion channel proteins are required for male fertility and sperm cell hyperactivated motility. Proc Natl Acad Sci U S A 104(4):1219–1223
Ren D et al (2001) A sperm ion channel required for sperm motility and male fertility. Nature 413(6856):603–609
Quill TA et al (2003) Hyperactivated sperm motility driven by CatSper2 is required for fertilization. Proc Natl Acad Sci U S A 100(25):14869–14874
Liu J et al (2007) CatSperbeta, a novel transmembrane protein in the CatSper channel complex. J Biol Chem 282(26):18945–18952
Wang H et al (2009) A novel, single, transmembrane protein CATSPERG is associated with CATSPER1 channel protein. Biol Reprod 81(3):539–544
Chung JJ et al (2011) A novel gene required for male fertility and functional CATSPER channel formation in spermatozoa. Nat Commun 2:153
Lishko PV, Botchkina IL, Kirichok Y (2011) Progesterone activates the principal Ca2+ channel of human sperm. Nature 471(7338):387–391
Strunker T et al (2011) The CatSper channel mediates progesterone-induced Ca2+ influx in human sperm. Nature 471(7338):382–386
Taussig LM et al (1972) Fertility in males with cystic fibrosis. N Engl J Med 287(12):586–589
Anguiano A et al (1992) Congenital bilateral absence of the vas deferens. A primarily genital form of cystic fibrosis. JAMA 267(13):1794–1797
Claustres M (2005) Molecular pathology of the CFTR locus in male infertility. Reprod Biomed Online 10(1):14–41
Reynaert I et al (2000) Morphological changes in the vas deferens and expression of the cystic fibrosis transmembrane conductance regulator (CFTR) in control, deltaF508 and knock-out CFTR mice during postnatal life. Mol Reprod Dev 55(2):125–135
O’Hara L et al (2011) Androgen receptor expression in the caput epididymal epithelium is essential for development of the initial segment and epididymal spermatozoa transit. Endocrinology 152(2):718–729
Krutskikh A et al (2011) Targeted inactivation of the androgen receptor gene in murine proximal epididymis causes epithelial hypotrophy and obstructive azoospermia. Endocrinology 152(2):689–696
Aston KI, Carrell DT (2009) Genome-wide study of single-nucleotide polymorphisms associated with azoospermia and severe oligozoospermia. J Androl 30(6):711–725
Aston KI et al (2010) Evaluation of 172 candidate polymorphisms for association with oligozoospermia or azoospermia in a large cohort of men of European descent. Hum Reprod 25(6):1383–1397
Summerer D (2009) Enabling technologies of genomic-scale sequence enrichment for targeted high-throughput sequencing. Genomics 94(6):363–368
Maxmen A (2011) Exome sequencing deciphers rare diseases. Cell 144(5):635–637
Meyerson M, Gabriel S, Getz G (2010) Advances in understanding cancer genomes through second-generation sequencing. Nat Rev Genet 11(10):685–696
Metzker ML (2010) Sequencing technologies- the next generation. Nat Rev Genet 11(1):31–46
Ostermeier GC et al (2002) Spermatozoal RNA profiles of normal fertile men. Lancet 360(9335):772–777
Yatsenko AN et al (2006) Non-invasive genetic diagnosis of male infertility using spermatozoal RNA: KLHL10 mutations in oligozoospermic patients impair homodimerization. Hum Mol Genet 15(23):3411–3419
Matzuk MM, Lamb DJ (2008) The biology of infertility: research advances and clinical challenges. Nat Med 14(11):1197–1213
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this protocol
Cite this protocol
Lin, YN., Matzuk, M.M. (2014). Genetics of Male Fertility. In: Rosenwaks, Z., Wassarman, P. (eds) Human Fertility. Methods in Molecular Biology, vol 1154. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-0659-8_2
Download citation
DOI: https://doi.org/10.1007/978-1-4939-0659-8_2
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-0658-1
Online ISBN: 978-1-4939-0659-8
eBook Packages: Springer Protocols