Abstract
With recent advances in cancer therapeutics, there is a great need for improved imaging methods for characterizing cancer onset and progression in a quantitative and actionable way. Collagen, the most abundant extracellular matrix protein in the tumor microenvironment (and the body in general), plays a multifaceted role, both hindering and promoting cancer invasion and progression. Collagen deposition can defend the tumor with immunosuppressive effects, while aligned collagen fiber structures can enable tumor cell migration, aiding invasion and metastasis. Given the complex role of collagen fiber organization and topology, imaging has been a tool of choice to characterize these changes on multiple spatial scales, from the organ and tumor scale to cellular and subcellular level. Macroscale density already aids in the detection and diagnosis of solid cancers, but progress is being made to integrate finer microscale features into the process. Here we review imaging modalities ranging from optical methods of second harmonic generation (SHG), polarized light microscopy (PLM), and optical coherence tomography (OCT) to the medical imaging approaches of ultrasound and magnetic resonance imaging (MRI). These methods have enabled scientists and clinicians to better understand the impact collagen structure has on the tumor environment, at both the bulk scale (density) and microscale (fibrillar structure) levels. We focus on imaging methods with the potential to both examine the collagen structure in as natural a state as possible and still be clinically amenable, with an emphasis on label-free strategies, exploiting intrinsic optical properties of collagen fibers.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Henke E, Nandigama R, Ergün S (2020) Extracellular matrix in the tumor microenvironment and its impact on cancer therapy. Front Mol Biosci 6:160
Chu GC, Kimmelman AC, Hezel AF et al (2007) Stromal biology of pancreatic cancer. J Cell Biochem 101:887–907
Bellizzi AM, Frankel WL (2009) Pancreatic pathology: a practical review. Lab Med 40:417–426
Sarantis P, Koustas E, Papadimitropoulou A et al (2020) Pancreatic ductal adenocarcinoma: treatment hurdles, tumor microenvironment and immunotherapy. World J Gastrointest Oncol 12:173–181
Ricard-Blum S (2011) The collagen family. Cold Spring Harb Perspect Biol 3:a004978
Arun Gopinathan P, Kokila G, Jyothi M et al (2015) Study of collagen birefringence in different grades of oral squamous cell carcinoma using Picrosirius red and polarized light microscopy. Scientifica 2015:1–7
Perry SW, Burke RM, Brown EB (2012) Two-photon and second harmonic microscopy in clinical and translational cancer research. Ann Biomed Eng 40:277–291
Goss SA, O’Brien WD (1979) Direct ultrasonic velocity measurements of mammalian collagen threads. J Acoust Soc Am 65:507–511
Kakkad S, Zhang J, Akhbardeh A et al (2016) Collagen fibers mediate MRI-detected water diffusion and anisotropy in breast cancers. Neoplasia 18:585–593
Hauge A, Wegner CS, Gaustad J-V et al (2017) Diffusion-weighted MRI-derived ADC values reflect collagen I content in PDX models of uterine cervical cancer. Oncotarget 8:105682–105691
Boyd N, Martin L, Chavez S et al (2009) Breast-tissue composition and other risk factors for breast cancer in young women: a cross-sectional study. Lancet Oncol 10:569–580
Chen Y, Kim J, Yang S et al (2021) Type I collagen deletion in αSMA+ myofibroblasts augments immune suppression and accelerates progression of pancreatic cancer. Cancer Cell 39:548–565
Klöppel G (2007) Chronic pancreatitis, pseudotumors and other tumor-like lesions. Mod Pathol 20:S113–S131
Mihaljevic AL, Esposito I, Friess H et al (2009) Molecular biology, models, and histopathology of chronic pancreatitis and pancreatic cancer. Eur Surg 41:250–267
Conklin MW, Eickhoff JC, Riching KM et al (2011) Aligned collagen is a prognostic signature for survival in human breast carcinoma. Am J Pathol 178:1221–1232
Brett EA, Sauter MA, Machens H-G et al (2020) Tumor-associated collagen signatures: pushing tumor boundaries. Cancer Metabolism 8:14
Xi G, Guo W, Kang D et al (2021) Large-scale tumor-associated collagen signatures identify high-risk breast cancer patients. Theranostics 11:3229–3243
Conklin MW, Gangnon RE, Sprague BL et al (2018) Collagen alignment as a predictor of recurrence after ductal carcinoma in situ. Cancer Epidemiol Biomark Prev 27:138–145
Drifka CR, Loeffler AG, Mathewson K et al (2016) Highly aligned stromal collagen is a negative prognostic factor following pancreatic ductal adenocarcinoma resection. Oncotarget 7:76197–76213
Ouellette JN, Drifka CR, Pointer KB et al (2021) Navigating the collagen jungle: the biomedical potential of fiber Organization in Cancer. Bioengineering (Basel) 8:17
Boyd NF (2013) Mammographic density and risk of breast cancer. Am Soc Clin Oncol Educ Book:e57–e62. https://doi.org/10.1200/EdBook_AM.2013.33.e57
Lee K, Park HY, Kim KW et al (2019) Advances in whole body MRI for musculoskeletal imaging: diffusion-weighted imaging. J Clin Orthop Trauma 10:680–686
Rasmuson A, Segerström L, Nethander M et al (2012) Tumor development, growth characteristics and Spectrum of genetic aberrations in the TH-MYCN mouse model of neuroblastoma. PLoS One 7:e51297
Sun Y, Wang J, Shi J et al (2021) Synthetic polarization-sensitive optical coherence tomography by deep learning. npj Digit Med 4:105
Keikhosravi A, Bredfeldt JS, Sagar AK et al (2014) Chapter 28: Second-harmonic generation imaging of cancer. In: Waters JC, Wittman T (eds) Methods in cell biology. Academic, pp 531–546
Leighton TG (2007) What is ultrasound? Prog Biophys Mol Biol 93:3–83
Denk W, Strickler JH, Webb WW (1990) Two-photon laser scanning fluorescence microscopy. Science 248:73–76
Szulczewski JM, Inman DR, Entenberg D et al (2016) In vivo visualization of stromal macrophages via label-free FLIM-based metabolite imaging. Sci Rep 6:25086
Pinkert MA, Simmons ZJ, Niemeier RC et al (2020) Platform for quantitative multiscale imaging of tissue composition. Biomed Opt Express 11:1927–1946
Humm JL, Rosenfeld A, Del Guerra A (2003) From PET detectors to PET scanners. Eur J Nucl Med Mol Imaging 30:1574–1597
Serai SD (2022) Basics of magnetic resonance imaging and quantitative parameters T1, T2, T2*, T1rho and diffusion-weighted imaging. Pediatr Radiol 52:217–227
Centonze VE, White JG (1998) Multiphoton excitation provides optical sections from deeper within scattering specimens than confocal imaging. Biophys J 75:2015–2024
Birk JW, Tadros M, Moezardalan K et al (2014) Second harmonic generation imaging distinguishes both high-grade dysplasia and cancer from Normal colonic mucosa. Dig Dis Sci 59:1529–1534
Fujimoto JG, Schmitt J, Swanson E et al (2020) The development of optical coherence tomography. In: Jang I-K (ed) Cardiovascular OCT imaging. Springer, Cham, pp 1–23
Mohler W, Millard AC, Campagnola PJ (2003) Second harmonic generation imaging of endogenous structural proteins. Methods 29:97–109
Poole JJA, Mostaço-Guidolin LB (2021) Optical microscopy and the extracellular matrix structure: a review. Cell 10:1760
Neal RD, Tharmanathan P, France B et al (2015) Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br J Cancer 112(Suppl 1):S92–S107
Hawkes N (2019) Cancer survival data emphasize importance of early diagnosis. BMJ 364:l408
Clark M, Ford C (2019) Smartphone-, tablet-, or app-based portable ultrasound: a review of clinical effectiveness. Canadian Agency for Drugs and Technologies in Health, Ottawa
Wen B, Campbell KR, Cox BL et al (2015) Multi-view second-harmonic generation imaging of mouse tail tendon via reflective micro-prisms. Opt Lett 40:3201–3204
Campbell KR, Wen B, Shelton EM et al (2017) 3D second harmonic generation imaging tomography by multi-view excitation. Optica 4:1171–1179
Pinkert MA, Salkowski LR, Keely PJ et al (2018) Review of quantitative multiscale imaging of breast cancer. J Med Imaging (Bellingham) 5:010901
Morse DL, Galons J-P, Payne CM et al (2007) MRI-measured water mobility increases in response to chemotherapy via multiple cell-death mechanisms. NMR Biomed 20:602–614
Partridge SC, Nissan N, Rahbar H et al (2017) Diffusion-weighted breast MRI: clinical applications and emerging techniques. J Magn Reson Imaging 45:337–355
Shiftan L, Israely T, Cohen M et al (2005) Magnetic resonance imaging visualization of hyaluronidase in ovarian carcinoma. Cancer Res 65:10316–10323
Nieminen MT, Rieppo J, Töyräs J et al (2001) T2 relaxation reveals spatial collagen architecture in articular cartilage: a comparative quantitative MRI and polarized light microscopic study. Magn Reson Med 46:487–493
Ross KA, Williams RM, Schnabel LV et al (2013) Comparison of three methods to quantify repair cartilage collagen orientation. Cartilage 4:111–120
Faragli A, Merz S, Muzio FPL et al (2020) Estimation of total collagen volume: a T1 mapping versus histological comparison study in healthy Landrace pigs. Int J Cardiovasc Imaging 36:1761–1769
Fullerton GD, Rahal A (2007) Collagen structure: the molecular source of the tendon magic angle effect. J Magn Reson Imaging 25:345–361
Xia Y (2000) Magic-angle effect in magnetic resonance imaging of articular cartilage: a review. Investig Radiol 35:602–621
Liu XS, Zhang XH, Rajapakse CS et al (2010) Accuracy of high-resolution in vivo micro magnetic resonance imaging for measurements of microstructural and mechanical properties of human distal tibial bone. J Bone Miner Res 25:2039–2050
Nowogrodzki A (2018) The world’s strongest MRI machines are pushing human imaging to new limits. Nature 563:24–26
Dietrich O, Reiser MF, Schoenberg SO (2008) Artifacts in 3-T MRI: physical background and reduction strategies. Eur J Radiol 65:29–35
Krupa K, Bekiesińska-Figatowska M (2015) Artifacts in magnetic resonance imaging. Pol J Radiol 80:93–106
Wald LL, McDaniel PC, Witzel T et al (2020) Low-cost and portable MRI. J Magn Reson Imaging 52:686–696
Hori M, Hagiwara A, Goto M et al (2021) Low-field magnetic resonance imaging. Investig Radiol 56:669–679
Leithner D, Moy L, Morris EA et al (2019) Abbreviated MRI of the breast: does it provide value? J Magn Reson Imaging 49:e85–e100
Caravan P, Das B, Dumas S et al (2007) Collagen-targeted MRI contrast agent for molecular imaging of fibrosis. Angew Chem Int Ed 46:8171–8173
Salarian M, Turaga RC, Xue S et al (2019) Early detection and staging of chronic liver diseases with a protein MRI contrast agent. Nat Commun 10:4777
Erstad DJ, Sojoodi M, Taylor MS et al (2020) Fibrotic response to neoadjuvant therapy predicts survival in pancreatic cancer and is measurable with collagen-targeted molecular MRI. Clin Cancer Res 26:5007–5018
Harel A, Eliav U, Akselrod S et al (2008) Magnetization transfer based contrast for imaging denatured collagen. J Magn Reson Imaging 27:1155–1163
Hodgson RJ, O’Connor PJ, Grainger AJ (2012) Tendon and ligament imaging. Br J Radiol 85:1157–1172
Seifert R, Kersting D, Rischpler C et al (2021) Clinical use of PET/MR in oncology: an update. Semin Nucl Med 52:356–364
Faria SC, Devine CE, Rao B et al (2019) Imaging and staging of endometrial cancer. Semin Ultrasound CT MRI 40:287–294
Klibanov AL, Hossack JA (2015) Ultrasound in radiology: from anatomic, functional, molecular imaging to drug delivery and image-guided therapy. Investig Radiol 50:657–670
Fields S, Dunn F (1973) Letter: correlation of echographic visualizability of tissue with biological composition and physiological state. J Acoust Soc Am 54:809–812
Tian L, Hunt B, Bell MAL et al (2021) Deep learning in biomedical optics. Lasers Surg Med 53:748–775
Ultrasound. https://www.nibib.nih.gov/science-education/science-topics/ultrasound. Accessed 12 Mar 2022
Official Statement. https://www.aium.org/officialStatements/39. Accessed 31 Mar 2022
Ultrasound for Cancer. https://www.cancer.org/treatment/understanding-your-diagnosis/tests/ultrasound-for-cancer.html. Accessed 12 Mar 2022
Fornage BD (1995) Role of color Doppler imaging in differentiating between pseudocystic malignant tumors and fluid collections. J Ultrasound Med 14:125–128
Pohlhammer J, O’Brien WD (1981) Dependence of the ultrasonic scatter coefficient on collagen concentration in mammalian tissues. J Acoust Soc Am 69:283–285
Inkinen S, Liukkonen J, Ylärinne JH et al (2014) Collagen and chondrocyte concentrations control ultrasound scattering in agarose scaffolds. Ultrasound Med Biol 40:2162–2171
Mercado KP, Helguera M, Hocking DC et al (2015) Noninvasive quantitative imaging of collagen microstructure in three-dimensional hydrogels using high-frequency ultrasound. Tissue Eng Part C Methods 21:671–682
Guerrero QW, Rosado-Mendez IM, Drehfal LC et al (2017) Quantifying backscatter anisotropy using the reference phantom method. IEEE Trans Ultrason Ferroelectr Freq Control 64:1063–1077
Guerrero QW, Feltovich H, Rosado-Mendez IM et al (2018) Anisotropy and spatial heterogeneity in quantitative ultrasound parameters: relevance to study of the human cervix. Ultrasound Med Biol 44:1493–1503
Guerrero QW, Feltovich H, Rosado-Mendez IM et al (2019) Quantitative ultrasound biomarkers based on backscattered acoustic power: potential for quantifying remodeling of the human cervix during pregnancy. Ultrasound Med Biol 45:429–439
Guerrero QW, Feltovich H, Rosado-Mendez I et al (2019) Quantitative ultrasound parameters based on the backscattered echo power signal as biomarkers of cervical remodeling: a longitudinal study in the pregnant rhesus macaque. Ultrasound Med Biol 45:1466–1474
Sarvazyan A, Hall TJ, Urban MW et al (2011) An overview of elastography – an emerging branch of medical imaging. Curr Med Imaging Rev 7:255–282
Wang ZL, Sun L, Li Y, Li N (2015) Relationship between elasticity and collagen fiber content in breast disease: a preliminary report. Ultrasonics 57:44–49
Riegler J, Labyed Y, Rosenzweig S et al (2018) Tumor Elastography and its association with collagen and the tumor microenvironment. Clin Cancer Res 24:4455–4467
Aumann S, Donner S, Fischer J et al (2019) Optical coherence tomography (OCT): principle and technical realization. In: Bille JF (ed) High resolution imaging in microscopy and ophthalmology: new frontiers in biomedical optics. Springer, Cham, pp 59–85
Huang D, Swanson EA, Lin CP et al (1991) Optical coherence tomography. Science 254:1178–1181
Swanson EA, Fujimoto JG (2017) The ecosystem that powered the translation of OCT from fundamental research to clinical and commercial impact [invited]. Biomed Opt Express 8:1638–1664
Popescu DP, Choo-Smith L-P, Flueraru C et al (2011) Optical coherence tomography: fundamental principles, instrumental designs and biomedical applications. Biophys Rev 3:155
Liu G, Lin AJ, Tromberg BJ et al (2012) A comparison of Doppler optical coherence tomography methods. Biomed Opt Express 3:2669–2680
Drexler W, Fujimoto JG (2015) Optical coherence tomography: technology and applications. Springer
Xu J, Song S, Wei W et al (2017) Wide field and highly sensitive angiography based on optical coherence tomography with akinetic swept source. Biomed Opt Express 8:420–435
Pfäffle C, Spahr H, Hillmann D et al (2017) Reduction of frame rate in full-field swept-source optical coherence tomography by numerical motion correction [invited]. Biomed Opt Express 8:1499–1511
Brand S, Poneros JM, Bouma BE et al (2000) Optical coherence tomography in the gastrointestinal tract. Endoscopy 32:796–803
Srinivasan VJ, Sakadžić S, Gorczynska I et al (2010) Quantitative cerebral blood flow with optical coherence tomography. Opt Express 18:2477–2494
Kim J, Brown W, Maher JR et al (2015) Functional optical coherence tomography: principles and progress. Phys Med Biol 60:R211–R237
de Boer JF, Hitzenberger CK, Yasuno Y (2017) Polarization sensitive optical coherence tomography – a review [Invited]. Biomed Opt Express 8:1838–1873
Gora MJ, Suter MJ, Tearney GJ et al (2017) Endoscopic optical coherence tomography: technologies and clinical applications [invited]. Biomed Opt Express 8:2405–2444
Wang J, Xu Y, Boppart SA (2017) Review of optical coherence tomography in oncology. J Biomed Opt 22:121711
Wang J, Xu Y, Mesa KJ et al (2018) Complementary use of polarization-sensitive and standard OCT metrics for enhanced intraoperative differentiation of breast cancer. Biomed Opt Express 9:6519–6528
Spaide RF, Fujimoto JG, Waheed NK et al (2018) Optical coherence tomography angiography. Prog Retin Eye Res 64:1–55
Hariri LP, Adams DC, Applegate MB et al (2019) Distinguishing tumor from associated fibrosis to increase diagnostic biopsy yield with polarization-sensitive optical coherence tomography. Clin Cancer Res 25:5242–5249
Oldenburg AL, Applegate BE, Izatt JA et al (2008) Molecular OCT contrast enhancement and imaging. In: Drexler W, Fujimoto JG (eds) Optical coherence tomography: technology and applications. Springer, Berlin/Heidelberg, pp 713–756
Skala MC, Crow MJ, Wax A et al (2008) Photothermal optical coherence tomography of epidermal growth factor receptor in live cells using immunotargeted gold nanospheres. Nano Lett 8:3461–3467
Tucker-Schwartz JM, Beavers KR, Sit WW et al (2014) In vivo imaging of nanoparticle delivery and tumor microvasculature with multimodal optical coherence tomography. Biomed Opt Express 5:1731–1743
Oldenburg AL, Toublan FJ-J, Suslick KS et al (2005) Magnetomotive contrast for in vivo optical coherence tomography. Opt Express 13:6597–6614
Crecea V, Ahmad A, Boppart SA (2013) Magnetomotive optical coherence elastography for microrheology of biological tissues. J Biomed Opt 18:121504
Kennedy KM, McLaughlin RA, Kennedy BF et al (2013) Needle optical coherence elastography for the measurement of microscale mechanical contrast deep within human breast tissues. J Biomed Opt 18:121510
Leartprapun N, Iyer RR, Untracht GR et al (2018) Photonic force optical coherence elastography for three-dimensional mechanical microscopy. Nat Commun 9:2079
Oldenburg AL, Xu C, Boppart SA (2007) Spectroscopic optical coherence tomography and microscopy. IEEE J Sel Top Quantum Electron 13:1629–1640
Robles FE, Wilson C, Grant G et al (2011) Molecular imaging true-colour spectroscopic optical coherence tomography. Nat Photon 5:744–747
Westphal V, Yazdanfar S, Rollins AM et al (2002) Real-time, high velocity-resolution color Doppler optical coherence tomography. Opt Lett 27:34–36
Baumann B (2017) Polarization sensitive optical coherence tomography: a review of technology and applications. Appl Sci 7:474
Tuchin VV (2016) Polarized light interaction with tissues. J Biomed Opt 21:071114
Li E, Makita S, Hong Y-J et al (2017) Three-dimensional multi-contrast imaging of in vivo human skin by Jones matrix optical coherence tomography. Biomed Opt Express 8:1290–1305
Walther J, Li Q, Villiger M et al (2019) Depth-resolved birefringence imaging of collagen fiber organization in the human oral mucosa in vivo. Biomed Opt Express 10:1942–1956
Fujimoto JG, Pitris C, Boppart SA et al (2000) Optical coherence tomography: an emerging technology for biomedical imaging and optical biopsy. Neoplasia 2:9–25
Smith BR, Gambhir SS (2017) Nanomaterials for in vivo imaging. Chem Rev 117:901–986
Tang P, Kirby MA, Le N et al (2021) Polarization sensitive optical coherence tomography with single input for imaging depth-resolved collagen organizations. Light Sci Appl 10:237
Li Q, Karnowski K, Untracht G et al (2020) Vectorial birefringence imaging by optical coherence microscopy for assessing fibrillar microstructures in the cornea and limbus. Biomed Opt Express 11:1122–1138
Vasquez D, Knorr F, Hoffmann F et al (2021) Multimodal scanning microscope combining optical coherence tomography, Raman spectroscopy and fluorescence lifetime microscopy for mesoscale label-free imaging of tissue. Anal Chem 93:11479–11487
Scolaro L, McLaughlin RA, Kennedy BF et al (2014) A review of optical coherence tomography in breast cancer. Photonics Lasers Med 3:225–240
Nolan RM, Adie SG, Marjanovic M et al (2016) Intraoperative optical coherence tomography for assessing human lymph nodes for metastatic cancer. BMC Cancer 16:144
Si P, Honkala A, Zerda A et al (2020) Optical microscopy and coherence tomography of cancer in living subjects. Trends Cancer 6:205–222
Yang VXD, Tang S, Gordon ML et al (2005) Endoscopic Doppler optical coherence tomography in the human GI tract: initial experience. Gastrointest Endosc 61:879–890
Yashin KS, Kiseleva EB, Gubarkova EV et al (2019) Cross-polarization optical coherence tomography for brain tumor imaging. Front Oncol 9:201
Park BH, Saxer C, Srinivas SM et al (2001) In vivo burn depth determination by high-speed fiber-based polarization sensitive optical coherence tomography. J Biomed Opt 6:474–479
Chen P-H, Lee H-Y, Chen Y-F et al (2020) Detection of Oral dysplastic and early cancerous lesions by polarization-sensitive optical coherence tomography. Cancers 12:2376
Duan L, Yamanari M, Yasuno Y (2012) Automated phase retardation oriented segmentation of chorio-scleral interface by polarization sensitive optical coherence tomography. Opt Express 20:3353–3366
Strasswimmer J, Pierce MC, Park BH et al (2004) Polarization-sensitive optical coherence tomography of invasive basal cell carcinoma. J Biomed Opt 9:292–298
Kim KH, Burns JA, Bernstein JJ et al (2010) In vivo 3D human vocal fold imaging with polarization sensitive optical coherence tomography and a MEMS scanning catheter. Opt Express 18:14644–14653
Adams DC, Szabari MV, Lagares D et al (2020) Assessing the progression of systemic sclerosis by monitoring the tissue optic axis using PS-OCT. Sci Rep 10:2561
Wang LV, Zimnyakov DA (2006) Optical polarization in biomedical applications. Springer
Kalwani NM, Ong CA, Lysaght AC et al (2013) Quantitative polarized light microscopy of unstained mammalian cochlear sections. J Biomed Opt 18:26021
Oldenbourg R (2013) Polarized light microscopy: principles and practice. Cold Spring Harb Protoc 2013:pdb.top078600. https://doi.org/10.1101/pdb.top078600
He C, He H, Chang J et al (2021) Polarization optics for biomedical and clinical applications: a review. Light Sci Appl 10:194
Drifka CR, Loeffler AG, Mathewson K et al (2016) Comparison of picrosirius red staining with second harmonic generation imaging for the quantification of clinically relevant collagen fiber features in histopathology samples. J Histochem Cytochem 64:519–529
Keikhosravi A, Liu Y, Drifka C et al (2017) Quantification of collagen organization in histopathology samples using liquid crystal based polarization microscopy. Biomed Opt Express 8:4243–4256
Keikhosravi A, Shribak M, Conklin MW et al (2021) Real-time polarization microscopy of fibrillar collagen in histopathology. Sci Rep 11:19063
Azzam RMA (2016) Stokes-vector and Mueller-matrix polarimetry [invited]. J Opt Soc Am A 33:1396–1408
Mehta SB, Shribak M, Oldenbourg R (2013) Polarized light imaging of birefringence and diattenuation at high resolution and high sensitivity. J Opt 15:094007
Wolman M, Kasten FH (1986) Polarized light microscopy in the study of the molecular structure of collagen and reticulin. Histochemistry 85:41–49
Rieppo J, Hallikainen J, Jurvelin JS et al (2008) Practical considerations in the use of polarized light microscopy in the analysis of the collagen network in articular cartilage. Microsc Res Tech 71:279–287
Changoor A, Tran-Khanh N, Méthot S et al (2011) A polarized light microscopy method for accurate and reliable grading of collagen organization in cartilage repair. Osteoarthr Cartil 19:126–135
Antonelli MR (2011) Biomedical applications of polarimetric imaging contrast. Initial studies for scattering media and human tissues. Citeseer
Ghosh N, Vitkin AI (2011) Tissue polarimetry: concepts, challenges, applications, and outlook. J Biomed Opt 16:110801
Park J, Lindberg A, Vizet J et al (2019) Cervical cancer diagnostics with a multispectral Mueller polarimetric colposcope. In: Clinical and preclinical optical diagnostics II (2019), paper 11073_9. Optica Publishing Group, p 11073_9
Gribble A, Pinkert MA, Westreich J et al (2019) A multiscale Mueller polarimetry module for a stereo zoom microscope. Biomed Eng Lett 9:339–349
Le DL, Nguyen DT, Le TH et al (2021) Characterization of healthy and cancerous human skin tissue utilizing Stokes–Mueller polarimetry technique. Opt Commun 480:126460
Oldenbourg R (1996) A new view on polarization microscopy. Nature 381:811–812
Shribak M (2015) Polychromatic polarization microscope: bringing colors to a colorless world. Sci Rep 5:17340
Oldenbourg R (2005) Polarization microscopy with the LC-PolScope. Live cell imaging: a laboratory manual. Cold Spring Harbour Laboratory Press, pp 205–237
Yang B, Brazile B, Jan N-J et al (2018) Structured polarized light microscopy for collagen fiber structure and orientation quantification in thick ocular tissues. J Biomed Opt 23:106001
Mazumder N, Qiu J, Foreman MR et al (2012) Polarization-resolved second harmonic generation microscopy with a four-channel Stokes-polarimeter. Opt Express 20:14090–14099
Samim M, Krouglov S, Barzda V (2016) Nonlinear Stokes-Mueller polarimetry. Phys Rev A 93:013847
Kontenis L, Samim M, Karunendiran A et al (2016) Second harmonic generation double stokes Mueller polarimetric microscopy of myofilaments. Biomed Opt Express 7:559–569
Cisek R, Joseph A, Harvey M et al (2021) Polarization-sensitive second harmonic generation microscopy for investigations of diseased collagenous tissues. Front Phys 9. https://doi.org/10.3389/fphy.2021.726996
Yang B, Lee P-Y, Hua Y et al (2020) Instant polarized light microscopy for imaging collagen microarchitecture and dynamics. J Biophotonics 14:e202000326
Guo S-M, Yeh L-H, Folkesson J et al (2020) Revealing architectural order with quantitative label-free imaging and deep learning. elife 9:e55502
Yeh L-H, Ivanov IE, Byrum JR et al (2021) uPTI: uniaxial permittivity tensor imaging of intrinsic density and anisotropy. https://doi.org/10.1101/2020.12.15.422951
Campagnola PJ, Millard AC, Terasaki M et al (2002) Three-dimensional high-resolution second-harmonic generation imaging of endogenous structural proteins in biological tissues. Biophys J 82:493–508
Pena A-M, Boulesteix T, Dartigalongue T et al (2005) Chiroptical effects in the second harmonic signal of collagens I and IV. J Am Chem Soc 127:10314–10322
Campagnola P (2011) Second harmonic generation imaging microscopy: applications to diseases diagnostics. Anal Chem 83:3224–3231
James DS, Campagnola PJ (2021) Recent advancements in optical harmonic generation microscopy: applications and perspectives. BME Front. https://doi.org/10.34133/2021/3973857
Su P-J, Chen W-L, Chen Y-F et al (2011) Determination of collagen nanostructure from second-order susceptibility tensor analysis. Biophys J 100:2053–2062
Campagnola PJ, Dong C-Y (2011) Second harmonic generation microscopy: principles and applications to disease diagnosis. Laser Photonics Rev 5:13–26
Masedunskas A, Milberg O, Porat-Shliom N et al (2012) Intravital microscopy: a practical guide on imaging intracellular structures in live animals. BioArchitecture 2:143–157
Latour G, Gusachenko I, Kowalczuk L et al (2012) In vivo structural imaging of the cornea by polarization-resolved second harmonic microscopy. Biomed Opt Express 3:1–15
Nadiarnykh O, LaComb RB, Brewer MA et al (2010) Alterations of the extracellular matrix in ovarian cancer studied by Second Harmonic Generation imaging microscopy. BMC Cancer 10:94
Nagatomi J, Ebong EE (2018) Mechanobiology handbook, 2nd edn. CRC Press
Brasselet S (2011) Polarization-resolved nonlinear microscopy: application to structural molecular and biological imaging. Adv Opt Photon 3:205
Tilbury K, Lien C-H, Chen S-J et al (2014) Differentiation of Col I and Col III isoforms in stromal models of ovarian cancer by analysis of second harmonic generation polarization and emission directionality. Biophys J 106:354–365
Nadiarnykh O, Campagnola PJ (2009) Retention of polarization signatures in SHG microscopy of scattering tissues through optical clearing. Opt Express 17:5794–5806
Campbell KR, Campagnola PJ (2017) Wavelength-dependent second harmonic generation circular dichroism for differentiation of Col I and Col III isoforms in stromal models of ovarian cancer based on intrinsic chirality differences. J Phys Chem B 121:1749–1757
Favreau PF, Deal JA, Harris B et al (2020) Label-free spectroscopic tissue characterization using fluorescence excitation-scanning spectral imaging. J Biophotonics 13:e201900183
In vivo fluorescence spectroscopy and imaging for oncological applications – Wagnieres – 1998 – Photochemistry and Photobiology – Wiley Online Library. https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1751-1097.1998.tb02521.x?sid=nlm%3Apubmed. Accessed 21 Mar 2022
Azaripour A, Lagerweij T, Scharfbillig C et al (2016) A survey of clearing techniques for 3D imaging of tissues with special reference to connective tissue. Prog Histochem Cytochem 51:9–23
Ariel P (2017) A beginner’s guide to tissue clearing. Int J Biochem Cell Biol 84:35–39
White JG, Amos WB, Fordham M (1987) An evaluation of confocal versus conventional imaging of biological structures by fluorescence light microscopy. J Cell Biol 105:41–48
Squirrell JM, Wokosin DL, White JG et al (1999) Long-term two-photon fluorescence imaging of mammalian embryos without compromising viability. Nat Biotechnol 17:763–767
Tserevelakis GJ, Filippidis G, Megalou EV et al (2011) Cell tracking in live Caenorhabditis elegans embryos via third harmonic generation imaging microscopy measurements. J Biomed Opt 16:046019
Campagnola PJ, Loew LM (2003) Second-harmonic imaging microscopy for visualizing biomolecular arrays in cells, tissues and organisms. Nat Biotechnol 21:1356–1360
Zoumi A, Yeh A, Tromberg BJ (2002) Imaging cells and extracellular matrix in vivo by using second-harmonic generation and two-photon excited fluorescence. PNAS 99:11014–11019
Clough M, Chen IA, Park S-W et al (2021) Flexible simultaneous mesoscale two-photon imaging of neural activity at high speeds. Nat Commun 12:6638
Demas J, Manley J, Tejera F et al (2021) High-speed, cortex-wide volumetric recording of neuroactivity at cellular resolution using light beads microscopy. Nat Methods 18:1103–1111
Scipioni L, Rossetta A, Tedeschi G et al (2021) Phasor S-FLIM: a new paradigm for fast and robust spectral fluorescence lifetime imaging. Nat Methods 18:542–550
Chen X, Nadiarynkh O, Plotnikov S et al (2012) Second harmonic generation microscopy for quantitative analysis of collagen fibrillar structure. Nat Protoc 7:654–669
Fast A, Lal A, Durkin AF et al (2020) Fast, large area multiphoton exoscope (FLAME) for macroscopic imaging with microscopic resolution of human skin. Sci Rep 10:18093
You S, Sun Y, Chaney EJ et al (2018) Slide-free virtual histochemistry (Part I): development via nonlinear optics. Biomed Opt Express 9:5240–5252
Pouli D, Balu M, Alonzo CA et al (2016) Imaging mitochondrial dynamics in human skin reveals depth-dependent hypoxia and malignant potential for diagnosis. Sci Transl Med 8:367ra169
Lukina MM, Dudenkova VV, Shimolina LE et al (2019) In vivo metabolic and SHG imaging for monitoring of tumor response to chemotherapy. Cytometry A 95:47–55
Williams C, Quinn KP, Georgakoudi I et al (2014) Young developmental age cardiac extracellular matrix promotes the expansion of neonatal cardiomyocytes in vitro. Acta Biomater 10:194–204
Sun S, Titushkin I, Cho M (2006) Regulation of mesenchymal stem cell adhesion and orientation in 3D collagen scaffold by electrical stimulus. Bioelectrochemistry 69:133–141
Reusch LM, Feltovich H, Carlson LC et al (2013) Nonlinear optical microscopy and ultrasound imaging of human cervical structure. J Biomed Opt 18:031110
Rezakhaniha R, Agianniotis A, Schrauwen JTC et al (2012) Experimental investigation of collagen waviness and orientation in the arterial adventitia using confocal laser scanning microscopy. Biomech Model Mechanobiol 11:461–473
Kartasalo K, Pölönen R-P, Ojala M et al (2015) CytoSpectre: a tool for spectral analysis of oriented structures on cellular and subcellular levels. BMC Bioinform 16:344
Marcotti S, de Freitas DB, Troughton LD et al (2021) A workflow for rapid unbiased quantification of fibrillar feature alignment in biological images. Front Comput Sci 3:745831
Steger C (1998) An unbiased detector of curvilinear structures. IEEE Trans Pattern Anal Mach Intell 20:113–125
Wershof E, Park D, Barry DJ et al (2021) A FIJI Macro for quantifying pattern in extracellular matrix. Life Sci Alliance 4:e202000880
Xu T, Vavylonis D, Tsai F-C et al (2015) SOAX: a software for quantification of 3D biopolymer networks. Sci Rep 5:9081
Liu Y, Eliceiri KW (2020) Quantifying fibrillar collagen organization with curvelet transform-based tools. J Vis Exp 11. https://doi.org/10.3791/61931
Preibisch S, Saalfeld S, Tomancak P (2009) Globally optimal stitching of tiled 3D microscopic image acquisitions. Bioinformatics 25:1463–1465
Hörl D, Rojas Rusak F, Preusser F et al (2019) BigStitcher: reconstructing high-resolution image datasets of cleared and expanded samples. Nat Methods 16:870–874
Chalfoun J, Majurski M, Blattner T et al (2017) MIST: accurate and scalable microscopy image stitching tool with stage modeling and error minimization. Sci Rep 7:4988
Muhlich J, Chen Y-A, Russell D et al (2021) Stitching and registering highly multiplexed whole slide images of tissues and tumors using ASHLAR software. Bioinformatics. https://doi.org/10.1101/2021.04.20.440625
Bankhead P, Loughrey MB, Fernández JA et al (2017) QuPath: open source software for digital pathology image analysis. Sci Rep 7:16878
Sofroniew N, Lambert T, Nunez-Iglesias J et al (2022) napari/napari: 0.4.15. Zenodo
Besson S, Leigh R, Linkert M et al (2019) Bringing open data to whole slide imaging. Digit Pathol 2019:3–10
Fernandez R, Moisy C (2021) Fijiyama: a registration tool for 3D multimodal time-lapse imaging. Bioinformatics 37:1482–1484
Chiaruttini N, Burri O, Haub P et al (2022) An open-source whole slide image registration workflow at cellular precision using Fiji, QuPath and Elastix. Front Comput Sci 3:780026
Klein S, Staring M, Murphy K et al (2010) Elastix: a toolbox for intensity-based medical image registration. IEEE Trans Med Imaging 29:196–205
Avants BB, Tustison NJ, Stauffer M et al (2014) The insight ToolKit image registration framework. Front Neuroinform 8:44
Keikhosravi A, Li B, Liu Y et al (2020) Intensity-based registration of bright-field and second-harmonic generation images of histopathology tissue sections. Biomed Opt Express 11:160–173
Haskins G, Kruger U, Yan P (2020) Deep learning in medical image registration: a survey. Mach Vis Appl 31:8
Hadsell R, Chopra S, LeCun Y (2006) Dimensionality reduction by learning an invariant mapping. In: 2006 IEEE computer society conference on computer vision and pattern recognition – volume 2 (CVPR’06). IEEE, New York, pp 1735–1742
van den Oord A, Li Y, Vinyals O (2019) Representation learning with contrastive predictive coding. arXiv:180703748 [cs, stat]
Pielawski N, Wetzer E, Öfverstedt J et al (2020) CoMIR: contrastive multimodal image representation for registration. arXiv:200606325 [cs, eess]
Kaji S, Kida S (2019) Overview of image-to-image translation by use of deep neural networks: denoising, super-resolution, modality conversion, and reconstruction in medical imaging. Radiol Phys Technol 12:235–248
Keikhosravi A, Li B, Liu Y et al (2020) Non-disruptive collagen characterization in clinical histopathology using cross-modality image synthesis. Commun Biol 3:414
Si L, Li N, Huang T et al (2022) Computational image translation from Mueller matrix polarimetry to bright-field microscopy. J Biophotonics 15:e202100242
Isola P, Zhu J-Y, Zhou T et al (2017) Image-to-image translation with conditional adversarial networks. In: 2017 IEEE Conference on Computer Vision and Pattern Recognition (CVPR). IEEE, Honolulu, pp 5967–5976
Zhu J-Y, Park T, Isola P et al (2017) Unpaired image-to-image translation using cycle-consistent adversarial networks. In: 2017 IEEE International Conference on Computer Vision (ICCV). IEEE, Venice, pp 2242–2251
Lu J, Öfverstedt J, Lindblad J et al (2021) Is image-to-image translation the panacea for multimodal image registration? A comparative study. arXiv:210316262 [cs, eess]
Durkee MS, Abraham R, Clark MR et al (2021) Artificial intelligence and cellular segmentation in tissue microscopy images. Am J Pathol 191:1693–1701
Scherf N, Huisken J (2015) The smart and gentle microscope. Nat Biotechnol 33:815–818
Cromey DW (2010) Avoiding twisted pixels: ethical guidelines for the appropriate use and manipulation of scientific digital images. Sci Eng Ethics 16:639–667
Stylianou A, Gkretsi V, Stylianopoulos T (2018) Atomic force microscopy nano-characterization of 3D collagen gels with tunable stiffness. MethodsX 5:503–513
Truong TV, Supatto W, Koos DS et al (2011) Deep and fast live imaging with two-photon scanned light-sheet microscopy. Nat Methods 8:757–760
Lin P-Y, Hwang S-PL, Lee C-H et al (2021) Two-photon scanned light sheet fluorescence microscopy with axicon imaging for fast volumetric imaging. J Biomed Opt 26:116503
Hanrahan N, Lane SIR, Johnson P et al (2020) Label-free and multimodal second harmonic generation light sheet microscopy. Biophysics. https://doi.org/10.1101/2020.09.07.284703
Liu JTC, Glaser AK, Bera K et al (2021) Harnessing non-destructive 3D pathology. Nat Biomed Eng 5:203–218
Loebinger MR, Kyrtatos PG, Turmaine M et al (2009) Magnetic resonance imaging of mesenchymal stem cells homing to pulmonary metastases using biocompatible magnetic nanoparticles. Cancer Res 69:8862–8867
Farrar CT, Gale EM, Kennan R et al (2018) CM-101: type I collagen–targeted MR imaging probe for detection of liver fibrosis. Radiology 287:581–589
Kennedy P, Taouli B (2020) Collagen-targeted MRI contrast agent for liver fibrosis detection. Nat Rev Gastroenterol Hepatol 17:201–202
Schroeder AB, Karim A, Ocotl E et al (2020) Optical imaging of collagen fiber damage to assess thermally injured human skin. Wound Repair Regen 28:848–855
Zackrisson S, van de Ven SMWY, Gambhir SS (2014) Light in and sound out: emerging translational strategies for photoacoustic imaging. Cancer Res 74:979–1004
Wray P, Lin L, Hu P et al (2019) Photoacoustic computed tomography of human extremities. J Biomed Opt 24:026003
Steinberg I, Huland DM, Vermesh O et al (2019) Photoacoustic clinical imaging. Photo-Dermatology 14:77–98
Park E, Lee Y-J, Lee C et al (2020) Effective photoacoustic absorption spectrum for collagen-based tissue imaging. J Biomed Opt 25:056002
Regensburger AP, Fonteyne LM, Jüngert J et al (2019) Detection of collagens by multispectral optoacoustic tomography as an imaging biomarker for Duchenne muscular dystrophy. Nat Med 25:1905–1915
Levental KR, Yu H, Kass L et al (2009) Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139:891–906
Maller O, Drain AP, Barrett AS et al (2021) Tumor-associated macrophages drive stromal cell-dependent collagen crosslinking and stiffening to promote breast cancer aggression. Nat Mater 20:548–559
Cox BL, Erickson-Bhatt S, Szulczewski JM et al (2019) A novel bioreactor for combined magnetic resonance spectroscopy and optical imaging of metabolism in 3D cell cultures. Magn Reson Med 81:3379–3391
You S, Tu H, Chaney EJ et al (2018) Intravital imaging by simultaneous label-free autofluorescence-multiharmonic microscopy. Nat Commun 9:2125
Chen S-Y (2009) In vivo virtual biopsy of human skin by using noninvasive higher harmonic generation microscopy. IEEE J Sel Top Quantum Electron 16:478–492. https://doi.org/10.1109/JSTQE.2009.2031987
Liao Y-H, Chen S-Y, Chou S-Y et al (2013) Determination of chronological aging parameters in epidermal keratinocytes by in vivo harmonic generation microscopy. Biomed Opt Express 4:77–88
You S, Sun Y, Chaney EJ et al (2018) Slide-free virtual histochemistry (Part II): detection of field cancerization. Biomed Opt Express 9:5253–5268
Datta R, Heaster TM, Sharick JT et al (2020) Fluorescence lifetime imaging microscopy: fundamentals and advances in instrumentation, analysis, and applications. J Biomed Opt 25:071203
Jiang Y, Tomov I, Wang Y et al (2004) Second-harmonic optical coherence tomography. Opt Lett 29:1090–1092
Applegate BE, Yang C, Rollins AM et al (2004) Polarization-resolved second-harmonic-generation optical coherence tomography in collagen. Opt Lett 29:2252–2254
Chong SP, Lai T, Zhou Y et al (2013) Tri-modal microscopy with multiphoton and optical coherence microscopy/tomography for multi-scale and multi-contrast imaging. Biomed Opt Express 4:1584–1594
Welge WA, DeMarco AT, Watson JM et al (2014) Diagnostic potential of multimodal imaging of ovarian tissue using optical coherence tomography and second-harmonic generation microscopy. J Med Imaging (Bellingham) 1:025501
Leitgeb RA, Baumann B (2018) Multimodal optical medical imaging concepts based on optical coherence tomography. Front Phys 6. https://doi.org/10.3389/fphy/2018.00114
Graf BW, Boppart SA (2012) Multimodal in vivo skin imaging with integrated optical coherence and multiphoton microscopy. IEEE J Sel Top Quantum Electron 18:1280–1286
Dones JM, Tanrikulu IC, Chacko JV et al (2019) Optimization of interstrand interactions enables burn detection with a collagen-mimetic peptide. Org Biomol Chem 17:9906–9912
Bueno JM, Ávila FJ, Martínez-Ojeda RM et al (2020) Adaptive-optics polarization-sensitive second harmonic generation microscopy. In: 2020 22nd International Conference on Transparent Optical Networks (ICTON), pp 1–4
McConnell G, Trägårdh J, Amor R et al (2016) A novel optical microscope for imaging large embryos and tissue volumes with sub-cellular resolution throughout. elife 5:e18659
Winter PW, York AG, Nogare DD et al (2014) Two-photon instant structured illumination microscopy improves the depth penetration of super-resolution imaging in thick scattering samples. Optica 1:181–191
Urban BE, Yi J, Chen S et al (2015) Super-resolution two-photon microscopy via scanning patterned illumination. Phys Rev E Stat Nonlinear Soft Matter Phys 91:042703
Johnson PB, Johnson PB, Karvounis A et al (2021) Superresolved polarization-enhanced second-harmonic generation for direct imaging of nanoscale changes in collagen architecture. Optica 8:674–685
Wang L, Zheng X, Zhou J et al (2022) Improvement in resolution of multiphoton scanning structured illumination microscopy via harmonics. Engineering. https://doi.org/10.1016/j.eng.2021.12.010
Majeed H (2018) Label-free breast histopathology using quantitative phase imaging. PhD thesis, University of Illinois at Urbana-Champaign
Fanous M, Keikhosravi A, Kajdacsy-Balla A et al (2020) Quantitative phase imaging of stromal prognostic markers in pancreatic ductal adenocarcinoma. Biomed Opt Express 11:1354–1364
Costantini I, Cicchi R, Silvestri L et al (2019) In-vivo and ex-vivo optical clearing methods for biological tissues: review. Biomed Opt Express 10:5251–5267
Alali S, Vitkin IA (2015) Polarized light imaging in biomedicine: emerging Mueller matrix methodologies for bulk tissue assessment. J Biomed Opt 20:061104
Han S, Makareeva E, Kuznetsova NV et al (2010) Molecular mechanism of Type I collagen Homotrimer resistance to mammalian collagenases. J Biol Chem 285:22276–22281
Börner K, Maru JT, Goldstone RL (2004) The simultaneous evolution of author and paper networks. PNAS 101:5266–5273
Karczewski KJ, Snyder MP (2018) Integrative omics for health and disease. Nat Rev Genet 19:299–310
Acknowledgments
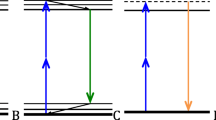
We thank Dr. Jayne Squirrell, Dr. Ellen Dobson, and Sam Griffin for very useful comments and edits on the manuscript. We acknowledge funding from NIH grants# U54CA268069 and R01CA238191. Figure 1 was created using BioRender.com.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Nelson, M.S. et al. (2023). Multiscale Label-Free Imaging of Fibrillar Collagen in the Tumor Microenvironment. In: Ursini-Siegel, J. (eds) The Tumor Microenvironment. Methods in Molecular Biology, vol 2614. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-2914-7_13
Download citation
DOI: https://doi.org/10.1007/978-1-0716-2914-7_13
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-2913-0
Online ISBN: 978-1-0716-2914-7
eBook Packages: Springer Protocols