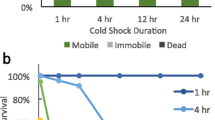
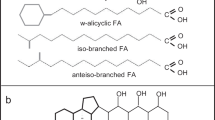
Abstract
The physiological adjustment of organisms in response to temperature variation is a crucial part of coping with environmental stress. An important component of the cold response is the increase in membrane lipid unsaturation, and this has been linked to an enhanced resistance to the debilitating or lethal effects of cold. Underpinning the lipid response is the upregulation of fatty acid desaturases (des), particularly those introducing double bonds at the 9–10 position of saturated fatty acids. For plants and microbes there is good genetic evidence that regulation of des genes, and the consequent changes in lipid saturation, are causally linked to generation of a cold-tolerant phenotype. In animals, however, supporting evidence is almost entirely limited to correlations of saturation with cold conditions. We describe our recent attempts to provide a direct test of this relationship by genetic manipulation of the nematode Caenorhabditis elegans. We show that this species displays a strong cold tolerant phenotype induced by prior conditioning to cold, and that this is directly linked to upregulated des activity. However, whilst genetic disruption of des activity and lipid unsaturation signiflcantly reduced cold tolerance, animals retained a substantial component of their stress tolerant phenotype produced by cold conditioning. This indicates that mechanisms other than lipid unsaturation play an important role in cold adaptation.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Cossins AR, Bowler K. The Temperature Biology of Animals. London: Chapman and Hall, 1987.
Hochachka PW, Somero GN. Biochemical adaptation. Princeton University Press, 2002.
Kültz D. Molecular and evolutionary basis of the cellular stress response. Annu Rev Physiol 2005;67:225–257.
Henriques V, Hansen C. Vergleichende Untersuchungen über die chemische Zusammenstzung des thierischen Fettes. Skand Arch Physiol 1901;11:151–165.
Cossins AR. The adaptation of membrane structure and function to changes in temperature. In: Cossins AR, Sheterline P, eds. Cellular acclimatisation to environmental change. Cambridge University Press, 1983:3–32.
Sinensky M. Homeoviscous adaptation-A homeostatic process that regulates the viscosity of membrane lipids in E. coli. Proc Nat Acad Sci USA 1974;71:522–525.
Hodkova M, Simek P, Zahradnickova H et al. Seasonal changes in the phospholipid composition in thoracic muscles of a heteropteran, Pyrrhocoris apterus. Insect Biochem Mol Biol 1999;29:367–376.
In: Aloia RC, Curtain CC, Gordon LM eds. Drug and anesthetic effects on membrane structure and function. New York: Wiley, 1991.
Heilbrunn LV. The colloid chemistry of protoplasm. IV. The heat coagulation of protoplasm. Am J Physiol 1924;69:190–199.
Suzuki I, Los DA, Kanesaki Y et al. The pathway for perception and transduction of low-temperature signals in Synechocystis. EMBO J 2000;19:1327–1334.
Orr GR, Raison JK. The effect of changing the composition of phosphatidylglycerol from thyla-koid polar lipids of oleander and cucumber on the temperature of the transition related to chilling injury. Planta 1990;181:137–143.
Cossins AR. Homeoviscous adaptation of biological membranes and its functional significance. In: Cossins AR, ed. Temperature adaptation of biological membranes. London: Portland Press, 1994:63–75.
Hazel JR, Landrey SR. Time course of thermal adaptation in plasma membranes of trout kidney. I. Headgroup composition. Am J Physiol Regul Int Comp Physiol 1988;255:R622–627.
Hazel JR, Zerba E. Adaptation of biological membranes to temperature: Molecular species compositions of phosphatidylcholine and phosphatidylethanolamine in mitochondrial and microso-mal membranes of liver from thermally-acclimated rainbow trout. J Comp Physiol B Biochem Syst Environ Physiol 1986;156:665–674.
Robertson JC, Hazel JR. Cholesterol content of trout plasma membranes varies with acclimation temperature. Am J Physiol Regul Int Comp Physiol 1995;269:R1113–1119.
Zehmer JK, Hazel JR. Thermally induced changes in lipid composition of raft and nonraft regions of hepatocyte plasma membranes of rainbow trout. J Exp Biol 2005;208:4283–4290.
Murata N, Suzuki I. Exploitation of genomic sequences in a systematic analysis to access how cyanobacteria sense environmental stress. J Exp Bot 2006;57:235–247.
Los DA, Murata N. Structure and expression of fatty acid desaturases. Biochim Biophys Acta 1998;1394:3–15.
Murata N, Wada H. Acyl-lipid desaturases and their importance in the tolerance and acclimatization to cold of cyanobacteria. Biochem J 1995;308:1–8.
Tasaka Y, Gombos Z, Nishiyama Y et al. Targeted mutagenesis of acyl-lipid desaturases in Synechocystis: Evidence for the important roles of polyunsaturated membrane lipids in growth, respiration and photosynthesis. Embo J 1996;15:6416–6425.
Sakamoto T, Shen GZ, Higashi S et al. Alteration of low-temperature susceptibility of the cyanobacterium Synechocystis sp. PCC 7002 by genetic manipulation of membrane lipid unsaturation. Arch Microbiol 1997;169:20–28.
Kanervo E, Tasaka Y, Murata N et al. Membrane lipid unsaturation modulates processing of the photosystem II reaction-center protein D1 at low temperatures. Plant Physiol 1997;114:841–849.
Wada H, Gombos Z, Murata N. Enhancement of chilling tolerance of a cyanobacterium by genetic manipulation of fatty acid desaturation. Nature 1990;347:200–203.
Vigh L, Los DA, Horvath I et al. The primary signal in the biological perception of temperature: Pd-catalysed hydrogenation of membrane lipids stimulates the expression of the desA gene in Synechocystis PCC 6803. Proc Nat Acad Sci USA 1993;90:9090–9094.
St. John JB, Christiansen MN, Ashworth EN et al. Effect of BASF 13-338, a substituted pyridazinone, on linolenic acid levels and winterhardiness of cereals. Crop Sci 1979;19:65–69.
Somerville C, Browse J. Plant lipids: Metabolism, mutants and membranes. Science 1991;252:80–87.
Browse J, Kunst L, Anderson S et al. A mutant of Arabidopsis deficient in the chloroplast 16:1/18:1 desaturase. Plant Physiol 1989;90:522–529.
Kunst L, Browse J, Somerville C. A mutant of Arabidopsis deficient in desaturation of palmitic acid in leaf lipids. Plant Physiol 1989;90:943–947.
Lemieux B, Miquel M, Somerville C et al. Mutants of Arabidopsis with alterations in seed lipid fatty acid composition. Theor Appl Genet 1990;80:234–240.
Hugly S, Somerville C. A role for membrane lipid polyunsaturation in chloroplast biogenesis at low temperature. Plant Physiol 1992;99:197–202.
Miquel M, James D, Dooner H et al. Arabidopsis requires polyunsaturated lipids for low-temperature survival. Proc Nat Acad Sci USA 1993;90:6208–6212.
Kodama H, Hamada T, Horiguchi G et al. Genetic enhancement of cold tolerance by expression of a gene for chloroplast ω-3 fatty acid desaturase in transgenic tobacco. Plant Physiol 1994;105:601–605.
Ishizaki-Nishizawa O, Fujii T, Azuma M et al. Low-temperature resistance of higher plants is significantly enhanced by nonspecific cyanobacterial desaturase. Nat Biotechnol 1996;14:1003–1006.
Orlova IV, Serebriiskaya TS, Popov V et al. Transformation of tobacco with a gene for the thermophilic acyl-lipid desaturase enhances the chilling tolerance of plants. Plant cell Physiol 2003;44:447–450.
Murata N, Ishizaki-Nishizawa O, Higashi S et al. Genetically engineered alteration in the chilling sensitivity of plants. Nature 1992;356:710–713.
Sakamoto A, Sulpice R, Hou CX et al. Genetic modification of the fatty acid unsaturation of phosphatidylglycerol in chloroplasts alters the sensitivity of tobacco plants to cold stress. Plant Cell Environ 2003;27:99–105.
Moon BY, Higashi S, Gombos Z et al. Unsaturation of the membrane lipids of chloroplasts stabilizes the photosynthetic machinery against low-temperature photoinhibition in transgenic tobacco plants. Proc Nat Acad Sci USA 1995;92:6219–6223.
Murakami Y, Tsuyama M, Kobayashi Y et al. Trienoic fatty acids and plant tolerance of high temperature. Science 2000;287:476–479.
Eguchi E, Ogawa Y, Okamoto K et al. Fatty acid compositions of arthropod and cephalopod photoreceptors-Interspecific, seasonal and development studies. J Comp Physiol B 1994;164:94–102.
Kostal V, Simek P. Changes in fatty acid composition of phospholipids and triacylglycerols after cold-acclimation of an aestivating insect prepupa. J Comp Physiol B 1998;168;453–460.
Cuculescu M, Pearson T, Hyde D et al. Heterothermal acclimation: An experimental paradigm for studying the control of thermal acclimation in crabs. Proc Nat Acad Sci USA 1999;96:6501–6505.
Lahdes E, Balogh G, Fodor E et al. Adaptation of composition and biophysical properties of phospholipids to temperature by the crustacean, Gammarus spp. Lipds 2000;35:1093–1098.
Bayley M, Peterson SO, Knigge T et al. Drought acclimation confers cold tolerance in the soil collembolan Folsomia Candida. J Insect Physiol 2001;47:1197–1204.
Hall JM, Parrish CC, Thompson RJ. Eicosapentaenoic acid regulates scallop (Placopecten magellanicus) membrane fluidity in response to cold. Biol bull 2002;202:201–203.
Cossins AR, Crawford DL. Fish as models for environmental genomics. Nat Rev Gen 2005;6:324–333.
Logue JA, de Vries AL, Fodor E et al. Lipid compositional correlates of temperature-adaptive interspecific differences in membrane physical structure. J Exp Biol 2000;203:2105–2115.
Wodtke E, Teichert T, Konig, A. Control of membrane fluidity in carp upon cold stress: Studies on fatty acid desaturases. In: Heller HC, ed. Living in the Cold: Physiological and Biochemical Adaptations. Elsevier, 1986:35–42.
Wodtke E, Cossins AR. Rapid cold-induced changes of membrane order and Δ9-desaturase activity in endoplasmic reticulum of carp liver: A time-course study of thermal acclimation. Biochim Biophys Acta 1991;1064:343–350.
Tiku PE, Gracey AY, Macartney AI et al. Cold-induced expression of Δ9-desaturase by transcrip-tional and post-translational mechanisms. Science 1996;271:815–818.
Polley SD, Tiku PE, Trueman RT et al. Differential expression of cold-and diet-specific genes encoding two carp liver Δ9-acyl-CoA desaturase isoforms. Am J Physiol 2003;284:R41–R50.
Gracey AY, Fraser EJ, Li W et al. Coping with cold: An integrative, multi-tissue analysis of the transcriptome of a poikilothermic vertebrate. Proc Nat Acad Sci USA 2004;101:6970–16975.
Greenberg AJ, Moran JR, Coyne JA et al. Ecological adaptation during incipient speciation revealed by precise gene replacement. Science 2003;302:1754–1757.
Overgaard J, Sørensen JG, Petersen SO et al. Changes in membrane lipid composition following rapid cold hardening in Drosophila melanogaster. J Insect Physiol 2005;51:1173–1182.
C. elegans II. Riddle DL, Blumenthal T, Meyer BJ, Priess JR, eds. Cold Spring Harbor Laboratory Press, 1997.
Fire A, Xu SQ, Montgomery MK et al. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998;391:806–811.
Murray PA, Hayward SAL, Gracey AY et al. Proc Natl Acad Sci USA, (In submission).
Phadtare S, Inouye M. Genome-wide transcriptional analysis of the cold shock response in wild-type and cold-sensitive, quadrupole-csp-deletion strains of Escherichia coli. J Bacteriol 2004;186:7007–7014.
Han Y, Zhou D, Pang X et al. DNA microarray analysis of the heat-and cold-shock stimulons in Yersinia pestis. Microb Infect 2005;7:335–348.
Qin W, Neal SJ, Robertson RM et al. Cold hardening and transcriptional change in Drosophila melanogaster. Insect Mol Biol 2005;14:607–613.
Hannah MA, Heyer AG, Hincha DK. A global survey of gene regulation during cold acclimation in Arabidopsis thaliana. PLoS Gen 2005;1:179–196.
Thieringer HA, Jones PG, Inouye M. Cold shock and adaptation. BioEssays 1998;20:49–57.
Schroder K, Zuber P, Willimsky G et al. Mapping of the Bacillus subtilis cspB gene and cloning of its homologs in thermophilic, mesophilic and psychrotropic bacilli. Gene 1993;136:277–280.
Wolffe AP. Structural and functional properties of the evolutionary ancient Y-box family of nucleic acid binding protein. BioEssays 1994;16:245–250.
Denlinger DL, Lee RE. Physiology of cold sensitivity. In: Hallman GJ, Denlinger DL, eds. Temperature sensitivity in insects and application in integrated pest management. Boulder: Westview Press, 1998:55–95.
Slachta M, Vambera J, Zahradnickova H et al. Entering diapause is a prerequisite for successful cold-acclimation in adult Graphosoma lineatum (Heteroptera: Pentatomidae). J Insect Physiol 2002;48:1031–1039.
Sabehat A, Weiss D, Lurie S. Heat-shock proteins and cross tolerance in plants. Physiol Plant 1998;103:437–441.
Carratù L, Franceschelli S, Pardini CL et al. Membrane lipid perturbation modifies the set point of the temperature of heat shock response in yeast. Proc Nat Acad Sci USA 1996;93:3870–3875.
Horváth I, Glatz A, Varvasovszki V et al. Membrane physical state controls the signaling mechanism of the heat shock response in Synechocystis PCC 6803: Identification of hspl7 as a “fluidity gene”. Proc Nat Acad Sci USA 1998;95:3513–3518.
Barua D, Heckathorn SA. Acclimation of the temperature set-points of the heat-shock response. J Therm Biol 2004;29:185–193.
Arispe N, Doh M, De Maio A. Lipid interaction differentiates the constitutive and stress-induced heat shock proteins Hsc70 and Hsp70. Cell Stress Chap 2002;7:330–338.
Vigh L, Maresca B, Harwood JL. Does the membrane’s physical state control the expression of heat shock and other genes? Trends Biochem Sci 1998;23:369–374.
Behm CA. The role of trehalose in the physiology of Nematodes. Int J Parasitol 1997;27:215–229.
Brown CR, Hong-Brown LQ, Doxsey SJ et al. Molecular chaperones and the centrosome. J Biol Chem 1996;271:833–840.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2007 Landes Bioscience and Springer Science+Business Media
About this chapter
Cite this chapter
Hayward, S.A.L., Murray, P.A., Gracey, A.Y., Cossins, A.R. (2007). Beyond the Lipid Hypothesis. In: Csermely, P., Vígh, L. (eds) Molecular Aspects of the Stress Response: Chaperones, Membranes and Networks. Advances in Experimental Medicine and Biology, vol 594. Springer, New York, NY. https://doi.org/10.1007/978-0-387-39975-1_12
Download citation
DOI: https://doi.org/10.1007/978-0-387-39975-1_12
Publisher Name: Springer, New York, NY
Print ISBN: 978-0-387-39974-4
Online ISBN: 978-0-387-39975-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)