Abstract
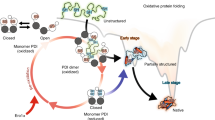
The folding of disulfide bond containing proteins proceeds in a biphasic manner. Initially, cysteines are oxidized to form disulfide bonds. Structure is largely absent during this phase. Next, when a minimally correct number of native linkages of disulfide bonds have been acquired, the biopolymer conformationally folds into the native, or a native-like, state. Thus, at the end of this “oxidative folding” process, a stable and biologically active protein is formed. This review focuses on dissecting the “structure-forming step” in oxidative protein folding. The ability to follow this pivotal step in protein maturation in somewhat detail is uniquely facilitated in “oxidative” folding scenarios. We review this step using bovine pancreatic Ribonuclease A as a model while recognizing the impact that this step has in subcellular trafficking and protein aggregation.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Abbreviations
- 3SNNU :
-
Unstructured three-disulfide-bond-containing intermediate with one or more non-native disulfide linkages
- 3SNU :
-
Unstructured three-disulfide-bond-containing intermediate with native disulfide linkages
- 3SNUx,y,z :
-
Unstructured three-disulfide-bond-containing intermediate with native disulfide linkages where x, y and z, refer to the cis- or trans- orientations of the X-Pro peptide bonds around prolines 93, 114 and 117, respectively
- Ux,y,z :
-
Unstructured disulfide-intact intermediate where x,y, and z refer to the cis- or trans- orientations of the X-Pro peptide bonds around prolines 93, 114 and 117, respectively
- Des species:
-
a folding intermediate lacking one disulfide bond
- DTTox :
-
Oxididized dithiothreitol
- DTTred :
-
Reduced Dithiothreitol
- ER:
-
Endoplamic Reticulum
- GSH:
-
Reduced glutathione
- GSSG:
-
Oxidized glutathione
- RNase A:
-
bovine pancreatic ribonuclease A
References
Arai K, Takei T, Okumura M, Watanabe S, Amagai Y, Asahina Y, Moroder L, Hojo H, Inaba K, Iwaoka M (2017) Preparation of Selenoinsulin as a long-lasting insulin analogue. Angew Chem Int Ed Engl 56:5522–5526
Arolas JL, Bronsoms S, Lorenzo J, Aviles FX, Chang JY, Ventura S (2004) Role of kinetic intermediates in the folding of leech carboxypeptidase inhibitor. J Biol Chem 279:37261–37270
Arolas JL, Aviles FX, Chang JY, Ventura S (2006) Folding of small disulfide-rich proteins: clarifying the puzzle. Trends Biochem Sci 31:292–301
Berkmen M (2012) Production of disulfide-bonded proteins in Escherichia Coli. Protein Expr Purif 82:240–251
Bulaj G, Olivera BM (2008) Folding of conotoxins: formation of the native disulfide bridges during chemical synthesis and biosynthesis of Conus peptides. Antioxid Redox Signal 10:141–155
Bulaj G, Buczek O, Goodsell I, Jimenez EC, Kranski J, Nielsen JS, Garrett JE, Olivera BM (2003) Efficient oxidative folding of conotoxins and the radiation of venomous cone snails. Proc Natl Acad Sci U S A 100(Suppl 2):14562–14568
de Marco A (2009) Strategies for successful recombinant expression of disulfide bond-dependent proteins in Escherichia Coli. Microb Cell Factories 14:8–26
Ewbank JJ, Creighton TE (1993a) Pathway of disulfide-coupled unfolding and refolding of bovine α-lactalbumin. Biochemistry 32:3677–3693
Ewbank JJ, Creighton TE (1993b) Structural characterization of the disulfide folding intermediates of bovine α-lactalbumin. Biochemistry 32:3694–3707
Gilbert HF (1990) Molecular and cellular aspects of thiol–disulfide exchange. In: Meister A (ed) Advances in enzymology and related areas of molecular biology, volume 63. Wiley, Hoboken
Hidaka Y, Shimamoto S (2013) Folding of peptides and proteins: role of disulfide bonds, recent developments. Biomol Concepts 4:597–604
Hidaka Y, Shimono C, Ohno M, Okumura N, Adermann K, Forssmann WG, Shimonishi Y (2000) Dual function of the propeptide of prouroguanylin in the folding of the mature peptide: disulfide-coupled folding and dimerization. J Biol Chem 275:25155–25162
Hober S, Uhlen M, Nilsson B (1997) Disulfide exchange folding of disulfide mutants of insulin-like growth factor I in vitro. Biochemistry 36:4616–4622
Houry WA, Scheraga HA (1996) Nature of the unfolded state of ribonuclease A: effect of cis-trans X-Pro peptide bond isomerization. Biochemistry 35:11719–11733
Houry WA, Rothwarf DM, Scheraga HA (1994) A very fast phase in the refolding of disulfide-intact ribonuclease A: implications for the refolding and unfolding pathways. Biochemistry 33:2516–2530
Houry WA, Rothwarf DM, Scheraga HA (1995) The nature of the initial step in the conformational folding of disulphide-intact ribonuclease A. Nat Struct Biol 2:495–503
Iwaoka M, Juminaga D, Scheraga HA (1998) Regeneration of three-disulfide mutants of bovine pancreatic ribonuclease a missing the 65−72 disulfide bond: characterization of a minor folding pathway of ribonuclease A and kinetic roles of Cys65 and Cys72. Biochemistry 37:4490–4501
Kauzmann W (1959) Relative probabilities of isomers in cystine-containing randomly coiled polypeptides. In: Benesch R, Benesc RE, Boyer PD, Klotz IM, Middlebrook WR, Szent-Györgyi AG, Schwarz DR (eds) Symposium on sulfur in proteins. Academic Press, New York. Chapter II.2
Ke N, Berkmen M (2014) Production of disulfide-bonded proteins in Escherichia Coli. Curr Protoc Mol Biol 108:16. 1B.1–21
Kubo S, Chino N, Kimura T, Sakakibara S (1996) Oxidative folding of omega-conotoxin MVIIC: effects of temperature and salt. Biopolymers 38:733–744
Milner SJ, Carver JA, Ballard FJ, Francis GL (1999) Probing the disulfide folding pathway of insulin-like growth factor-I. Biotechnol Bioeng 62:693–703
Narayan M (2011) The case of oxidative folding of ribonuclease A: factors impacting fold maturation of ER-processed proteins: the case of oxidative folding of ribonuclease A. In: Chang RJY, Ventura S (eds) Folding of disulfide proteins, Protein reviews (Zouhair Atassi M). Springer. http://link.springer.com/book/10.1007%2F978-1-4419-7273-6#page-1
Narayan M, Welker E, Wedemeyer WJ, Scheraga HA (2000) Oxidative folding of proteins. Acc Chem Res 33:805–812
Narayan M, Welker E, Scheraga HA (2001) Development of a novel method to study the rate-determining step during protein regeneration: application to the oxidative folding of RNase A at low temperature reveals BPTI-like kinetic traps. J Am Chem Soc 123:2909–2910
Navon A, Ittah V, Landsman P, Scheraga HA, Haas E (2001) Distributions of intramolecular distances in the reduced and denatured states of bovine pancreatic ribonuclease A. Folding initiation structures in the C-terminal portions of the reduced protein. Biochemistry 40:105–118
Oas TG, Kim PS (1988) A peptide model of a protein folding intermediate. Nature 336:42–48
Pace CN, Creighton TE (1986) The disulphide folding pathway of ribonuclease T1. J Mol Biol 188:477–486
Pace CN, Grimsley GR, Thomson JA, Barnett BJ (1988) Conformational stability and activity of Ribonuclease T1 with zero, one, and two intact disulfide bonds. J Biol Chem 263:11820–11825
Poland DC, Scheraga HA (1965) Statistical mechanics of noncovalent bonds in Polyamino acids. VIII covalent loops in proteins. Biopolymers 3:379–399
Radford SE, Dobson CM, Evans PA (1992) The folding of hen lysozyme involves partially structured intermediates and multiple pathways. Nature 358:302–307
Rothwarf DM, Scheraga HA (1993a) Regeneration of bovine pancreatic ribonuclease A. 1. Steady-state distribution. Biochemistry 32:2671–2679
Rothwarf DM, Scheraga HA (1993b) Regeneration of bovine pancreatic ribonuclease A. 2. Kinetics of regeneration. Biochemistry 32:2680–2689
Rothwarf DM, Scheraga HA (1993c) Regeneration of bovine pancreatic ribonuclease A. 3. Dependence on the nature of the redox reagent. Biochemistry 32:2690–2697. Erratum in: Biochemistry 1993 32:7064
Rothwarf DM, Scheraga HA (1993d) Regeneration of bovine pancreatic ribonuclease A. 4. Temperature dependence of the regeneration rate. Biochemistry 32:2698–2703
Rothwarf DM, Li YJ, Scheraga HA (1998a) Regeneration of bovine pancreatic ribonuclease A: identification of two nativelike three-disulfide intermediates involved in separate pathways. Biochemistry 37:3760–3766
Rothwarf DM, Li YJ, Scheraga HA (1998b) Regeneration of bovine pancreatic ribonuclease A: detailed kinetic analysis of two independent folding pathways. Biochemistry 37:3767–3776
Roux P, Ruoppolo M, Chaffotte A-F, Goldberg M (1999) Comparison of the kinetics of SS-bond, secondary structure, and active site formation during refolding of reduced denatured hen egg white lysozyme. Protein Sci 8:2751–2760
Stewart-Jones GB, Thomas PV, Chen M, Druz A, Joyce MG, Kong WP, Sastry M, Soto C, Yang Y, Zhang B, Chen L, Chuang GY, Georgiev IS, McLellan JS, Srivatsan S, Zhou T, Baxa U, Mascola JR, Graham BS, Kwong PD (2015) A cysteine zipper stabilizes a pre-fusion F glycoprotein vaccine for respiratory syncytial virus. PLoS One 10:e0128779
Thornton JM (1981) Disulphide bridges in globular proteins. J Mol Biol 151:261–287
van den Berg B, Chung EW, Robinson CV, Mateo PL, Dobson CM (1999) The oxidative refolding of hen lysozyme and its catalysis by protein disulfide isomerase. EMBO J 18:4794–4803
Volles MJ, Xu X, Scheraga HA (1999) Distribution of disulfide bonds in the two-disulfide intermediates in the regeneration of bovine pancreatic ribonuclease A: further insights into the folding process. Biochemistry 38:7284–7293
Wedemeyer WJ, Welker E, Narayan M, Scheraga HA (2000) Disulfide bonds and protein folding. Biochemistry 39:4207–4216
Weissman JS, Kim PS (1995) A kinetic explanation for the rearrangement pathway of BPTI folding. Nat Struct Biol 2:1123–1130
Welker E, Narayan M, Volles MJ, Scheraga HA (1999) Two new structured intermediates in the oxidative folding of RNase A. FEBS Lett 460:477–479
Welker E, Narayan M, Wedemeyer WJ, Scheraga HA (2001a) Structural determinants of oxidative folding in proteins. Proc Natl Acad Sci U S A 98:2312–2316
Welker E, Wedemeyer WJ, Narayan M, Scheraga HA (2001b) Coupling of conformational folding and disulfide-bond reactions in oxidative folding of proteins. Biochemistry 40:9059–9064
Wlodawer A, Svensson LA, Sjölin L, Gilliland GL (1988) Structure of phosphate-free ribonuclease A refined at 1.26 A. Biochemistry 27:2705–2717
Xu X, Scheraga HA (1998) Kinetic folding pathway of a three-disulfide mutant of bovine pancreatic ribonuclease A missing the [40–95] disulfide bond. Biochemistry 37:7561–7571
Xu X, Rothwarf DM, Scheraga HA (1996) Nonrandom distribution of the one-disulfide intermediates in the regeneration of ribonuclease A. Biochemistry 35:6406–6417
Yang Y, Wu J, Watson JT (1999) Probing the folding pathways of long R(3) insulin-like growth factor-I (LR(3)IGF-I) and IGF-I via capture and identification of disulfide intermediates by cyanylation methodology and mass spectrometry. J Biol Chem 274:37598–37604
Yu MH, Weissman JS, Kim PS (1995) Contributions of individual side-chains to the stability of BPTI examined by alanine-scanning mutagenesis. J Mol Biol 249:388–397
Acknowledgement
MN acknowledges NIH 1SC3 GM111200 01A1. The author wishes to thank Ms. Brenda Rubi Torres for creating some of the artwork in this chapter and Mr. Gyan M. Narayan for help with the manuscript. This work, is in part, an outcome of pedagogical techniques used by the author for a Biophysical Chemistry class taught by him in Spring 2016 (CHEM 4335). MN would like to acknowledge Jose A. Barragan, Homero R. Garcia, Natalia Luna, Vanessa I. Navarro, Stefani Perez Torres, and Alejandro Rodriguez for engaging in scintillating discussions on the subject of “The Structure-Forming Juncture in Oxidative Protein Folding: What happens in the ER?”
This work is in Compliance with Ethical Standards.
Conflicts of Interest
The author declares no conflicts of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Narayan, M. (2017). The Structure-Forming Juncture in Oxidative Protein Folding: What Happens in the ER?. In: Atassi, M. (eds) Protein Reviews. Advances in Experimental Medicine and Biology(), vol 966. Springer, Singapore. https://doi.org/10.1007/5584_2017_88
Download citation
DOI: https://doi.org/10.1007/5584_2017_88
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-6921-5
Online ISBN: 978-981-10-6922-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)