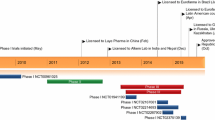
Abstract
Trelagliptin (Zafatek®) is an orally active dipeptidyl peptidase (DPP)-4 inhibitor developed by Takeda and approved in Japan for the treatment of type 2 diabetes mellitus (T2DM). Unlike other approved agents of its class, which are usually administered once daily, trelagliptin can be administered once weekly. Phase II development of trelagliptin was discontinued in the USA and EU, as Takeda considered that the costs associated with obtaining approval in these markets were prohibitive. This article summarizes the milestones in the development of trelagliptin leading to this first approval for T2DM.
Similar content being viewed by others
References
Takeda. Zafatek (trelagliptin): Japanese prescribing information. 2015. http://www.takedamed.com/content/medicine/pdf/zafatek.pdf. Accessed 27 May 2015.
Inzucchi SE, Bergenstal R, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes, 2015: a patient-centred approach. Update to a Position Statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. 2015;58:429–42.
Cramer JA. A systematic review of adherence with medications for diabetes. Diabetes Care. 2004;27:1218–24.
Kruk ME, Schwalbe N. The relation between intermittent dosing and adherence: preliminary insights. Clin Ther. 2006;28:1989–95.
PPD Inc. Takeda aquires the rights to DPP4 inhibitors granted to PPD [media release]. 13 July 2005. www.ppdi.com.
Actavis Inc. Actavis completes acquisition of Furiex Pharmaceuticals [media release]. 2 July 2014. www.actavis.com.
Drucker DJ. The role of gut hormones in glucose homeostasis. J Clin Invest. 2007;117:24–32.
Inagaki N, Onouchi H, Sano H, et al. SYR-472, a novel once-weekly dipeptidyl peptidase-4 (DPP-4) inhibitor, in type 2 diabetes mellitus: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2014;2:125–32.
Inagaki N, Onouchi H, Maezawa H, et al. Once-weekly trelagliptin versus daily alogliptin in Japanese patients with type 2 diabetes: a randomised, double-blind, phase 3, non-inferiority study. Lancet Diabetes Endocrinol. 2015;3:191–7.
Disclosure
The preparation of this review was not supported by any external funding. During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. K. McKeage is a salaried employee of Adis, Springer SBM.
Author information
Authors and Affiliations
Corresponding author
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Rights and permissions
About this article
Cite this article
McKeage, K. Trelagliptin: First Global Approval. Drugs 75, 1161–1164 (2015). https://doi.org/10.1007/s40265-015-0431-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-015-0431-9