Abstract
Background
The Urticaria Activity Score summed over 7 days (UAS7) assesses the itch severity and hive count in chronic spontaneous urticaria (CSU) using once- or twice-daily diary-based documentation.
Objective
The aim of this study was to evaluate the comparability of twice-daily versus once-daily versions of the UAS and the resulting UAS7 values.
Methods
Data came from the ASSURE-CSU study. The twice-daily and once-daily UAS7 was calculated from morning and evening ratings, as well as from exact 24-h evening ratings of hive count and itch severity, respectively. Three UAS7 scores were computed: UAS7 twice daily (UAS7TD), UAS7 once daily for maximum itch (UAS7OD1MAX), and UAS7 once daily for average itch (UAS7OD2AVG). UAS7 values were assigned to five score bands (0, 1–6, 7–15, 16–27, 28–42), reflecting urticaria-free to severe disease activity. The score values and score band ratios of the UAS7TD and UAS7OD versions were compared and assessed for correlation by weighted Cohen’s kappa statistics.
Results
Data from 614 patients were analyzed. All three versions of the UAS7 yielded very similar results, with a mean (standard deviation) UAS7TD, UAS7OD1MAX, and UAS7OD2AVG of 17.3 (10.49), 17.7 (8.90), and 16.2 (8.68), respectively. Correlation coefficients between UAS7TD and UAS7OD1MAX, UAS7TD and UAS7OD2AVG, and UAS7OD1MAX and UAS7OD2AVG were 0.94, 0.95, and 0.99, respectively, showing very high positive pairwise correlation. The weighted kappa coefficient, κ (95% confidence interval) was 0.78 (0.75–0.82) for UAS7TD versus UAS7OD1MAX, and 0.82 (0.78–0.85) for UAS7TD versus UAS7OD2AVG, demonstrating substantial agreement.
Conclusions
The once- and twice-daily UAS7 scores were highly consistent, supporting the use of either version when evaluating CSU activity.
Similar content being viewed by others
The Urticaria Activity Score (UAS) is a commonly used diary-based patient-reported outcome measure that assesses itch severity and hive count in chronic spontaneous urticaria (CSU), using once- or twice-daily diary-based documentation. |
The current study aimed to evaluate the comparability of twice-daily versus once-daily versions of the UAS and the resulting UAS7 values using data from a multinational, non-interventional study. |
The study found that weekly UAS-based measurements of disease activity in CSU by twice-daily or once-daily assessments were highly consistent and yielded very similar results. |
The findings provide evidence to support the use of either of the UAS versions when evaluating CSU activity as long as the same version is used over time for the same patients within a specific study or clinical practice. |
1 Introduction
Chronic spontaneous urticaria (CSU), formerly also known as chronic idiopathic urticaria (CIU), is characterized by the occurrence of wheals/hives, angioedema, or both for 6 weeks or longer in the absence of a specific trigger [1]. Chronic urticaria (CU) affects 0.5–1% of the general population worldwide [2], with CSU accounting for more than two-thirds of CU cases [3]. CSU affects several domains of health-related quality of life (HRQOL), such as activities of daily living, sleep, emotional and psychological well-being, and work productivity [4, 5]. Several types of patient-reported outcome (PRO) instruments, such as the EQ-5D (generic) [6], Dermatology Life Quality Index [DLQI] (dermatological) [7], and disease-specific Chronic Urticaria Quality of Life Questionnaire (CU-Q2oL) [8], have been used to assess quality of life and disease burden in CSU patients. The inclusion of PRO instruments in clinical practice is increasingly recommended because it allows assessment of the patients’ view of their disease and the effect of treatment. Additionally, it can help inform treatment decisions and improve physician–patient communication [9, 10]. This is even more relevant in CSU because symptoms are unpredictable and vary over time.
The Urticaria Activity Score (UAS) is a commonly used diary-based PRO measure that assesses the key sign (hives) and symptom (itch) of CSU. The international EAACI/GA2LEN/EDF/WAO guidelines for urticaria recommend use of the UAS in clinical practice to determine disease activity and response to treatment [1]. Currently, two versions of the daily UAS exist—one that assesses the number of hives and the intensity of itch twice daily (every 12 h), and one that assesses hive number and itch intensity once daily (every 24 h). Both versions can generate a weekly score (UAS7), calculated as the sum of the daily number of hives score and the itch severity score over 7 days. UAS7 values range from 0 to 42, with higher values reflecting higher disease activity [11, 12]. The twice-daily UAS [12, 13] has been accepted by the US FDA as a PRO measure supporting a label claim for drugs in CSU, and validated as per FDA guidelines for PRO instruments [14]. Although it is more burdensome for patients, the twice-daily assessment can capture more time-of-day nuances of disease activity. On the other hand, the once-daily diary [11] has been recommended by the EAACI/GA2LEN/EDF/WAO international urticaria guidelines [1], and has less patient burden of administration; however, it may be more prone to recall bias compared with the twice-daily UAS. No publications resulting from qualitative or quantitative work are currently available to confirm the validity of the once-daily version and the detailed instructions for its completion by patients. Additionally, different score ranges are used for the number of hives between the once-daily and twice-daily UAS questionnaires. It is thus important to understand whether the two UAS versions capture the same levels of disease activity. The objective of the present study was to evaluate the comparability of UAS7 scores obtained from the once-daily versus twice-daily versions of the UAS.
2 Methods
2.1 Data Source
Data came from the ASSURE-CSU study [15, 16], the first international study to quantify the humanistic and economic burden of illness of patients with CSU who are symptomatic despite treatment. This non-interventional, multinational study was conducted across seven countries (Canada, France, Germany, Italy, The Netherlands, Spain and the UK). Details of the study design have been previously published [15, 16] and are summarized below.
2.2 Study Sample and Design
The study enrolled adult patients (aged ≥ 18 years) with a clinician-confirmed, guideline-defined diagnosis of CSU who had been symptomatic for more than 12 months at least 3 days per week and were currently symptomatic despite treatment. The study included a 1-year retrospective medical record abstraction, a cross-sectional PRO survey, and a 7-day prospective urticaria patient daily diary (UPDD). The twice-daily version of the UAS diary was part of the UPDD and included morning and evening ratings of hives and itch severity (Table 1). Patients were asked the following question to report their itch intensity and hive count: “Thinking about the past 12 hours, please record the severity of itch and the number of hives you have, associated with your skin condition”. In addition, the exact 24-h hive counts was reported by patients in the UAS diary each evening (Table 1). These data were then used to compute the following three UAS7 scores: UAS7 twice daily (UAS7TD), UAS7 once daily for maximum itch (UAS7OD1MAX), and UAS7 once daily for average itch (UAS7OD2AVG) (Table 2). It is not known whether patients consider the maximum or average itch when reporting itch severity; therefore, both maximum and average daily itch severity scores were computed in the current study prior to calculating a daily score.
2.3 Calculation of Urticaria Activity Score over 7 Days (UAS7)
Table 2 presents the calculation of scores for the three scoring methods, i.e. UAS7TD, UAS7OD1MAX and UAS7OD2AVG. The UAS7TD method averaged each day’s morning and evening hive scores and itch scores respectively and then added the average daily hive and itch score values over 7 days. The UAS7OD1MAX method evaluated maximum itch and was calculated as the sum total of each day’s hive score (derived from an exact 24-h hive count) and each day’s maximum itch severity score (between morning and evening), over 7 days. The UAS7OD2AVG method evaluated average itch score and was calculated as the sum of each day’s hive score values (derived from an exact 24-h hive count) and each day’s average itch severity scores over 7 days.
UAS7 values were assigned to five score ranges (bands), reflecting urticaria-free to severe disease activity, measured by the weekly UAS7 as follows: UAS7 = 28–42, severe activity CSU; UAS7 = 16–27, moderate activity CSU; UAS7 = 7–15, mild activity CSU; UAS7 = 1–6, well-controlled CSU; UAS7 = 0, urticaria-free (Table 3) [17].
2.4 Statistical Analysis
Descriptive statistics were used to compute the overall mean UAS7 values, which were stratified into the abovementioned score ranges (UAS7: 0, 1–6, 7–15, 16–27 and 28–42) describing the disease activity. Pearson correlation coefficients were computed pairwise between the three UAS7 methods (UAS7TD, UAS7OD1MAX, and UAS7OD2AVG) and other PROs (DLQI, CU-Q2oL, EQ-5D). Scatterplots were used to understand the clustering of score values in patients who exhibited mismatches between score bands, from UAS7TD versus UAS7OD1MAX, and UAS7TD versus UAS7OD2AVG. Weighted Cohen’s kappa coefficients (κ) were computed to measure the agreement of disease activity score band assignments based on UAS7TD versus UAS7OD1MAX, and UAS7TD versus UAS7OD2AVG. A κ value of 0.61–0.80 denotes substantial agreement between the scores, whereas a κ value in the range of 0.81–1.00 indicates almost perfect agreement [18].
3 Results
3.1 Study Sample Characteristics
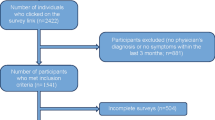
A total of 673 patients from 64 centers across seven countries were included in the study. The majority of enrolled patients were female (72.7%) and White/Caucasian (90.4%). The mean (standard deviation [SD]) age of patients at enrollment was 48.8 years (15.47).
Medical records were abstracted for all 673 patients, of whom 614 (91.2%) returned the 7-day UAS questionnaire. Of these 614 patients, 605 (98.5%) had non-missing values for UAS7TD, and 504 (82.1%) had non-missing values for UAS7OD1MAX and UAS7OD2AVG. The concordance analyses were restricted to 501 (81.6%) patients with non-missing UAS7 values for all three scoring methods (i.e. UAS7TD, UAS7OD1MAX, and UAS7OD2AVG).
3.2 All Versions of the UAS Yielded Similar UAS7 Score Values
All three versions of the UAS7, i.e. UAS7TD, UAS7OD1MAX, and UAS7OD2AVG, yielded very similar results, with a mean (SD) score value of 17.3 (10.49), 17.7 (8.90), and 16.2 (8.68), respectively (Fig. 1a, b). As expected, the overall mean (SD) weekly itch score values based on the daily maximum of twice-daily ratings [9.9 (5.28)] was higher than that based on the daily average of twice-daily ratings [8.4 (4.97)]. The mean (SD) weekly hive score values based on the daily average of twice-daily ratings was slightly higher [9.0 (6.58)] than that based on exact 24-h hive counts [7.7 (5.04)].
3.3 Pairwise Correlation among UAS7TD, UAS7OD1MAX, and UAS7OD2AVG
Of the 501 patients with complete data for the three scores, the correlation coefficients between UAS7TD and UAS7OD1MAX, UAS7TD and UAS7OD2AVG, and UAS7OD1MAX and UAS7OD2AVG were 0.94, 0.95, and 0.99, respectively, indicating very high positive pairwise correlation (Table 4). The pattern of correlation between each UAS7 version and other PRO measures was also consistent. For example, the correlation coefficient of UAS7TD, UAS7OD1MAX and UAS7OD2AVG to CU-Q2oL total score was 0.44, 0.45, and 0.44, respectively, and 0.45, 0.47 and 0.46, respectively, to DLQI total score (Table 4).
3.4 Concordance and Agreement Across UAS7 Scoring Methods
3.4.1 UAS7TD Versus UAS7OD1MAX
Of the patients with complete data for all three scores (N = 501), 388 (77.4%) were assigned to the same disease activity score band for UAS7TD versus UAS7OD1MAX (electronic supplementary Table S1). Additionally, of the 113 (22.6%) patients with different disease activity score band assignments based on UAS7TD versus UAS7OD1MAX, the majority (111/113 [98.2%]) were classified in adjacent score bands very close to the respective thresholds. Among these patients, the most commonly observed mismatches between UAS7TD and UAS7OD1MAX disease activity bands were seen in 36 (7.2%) patients classified in the 7–15 band based on UAS7TD, but with the UAS7OD1MAX in the higher activity band (16–27), and in 34 (6.8%) patients classified in the 28–42 band with UAS7TD, but with UAS7OD1MAX in the lower disease activity band (16–27). Results from scatterplots indicated that the UAS7TD and UAS7OD1MAX values for these patients tended to cluster near the threshold values for each score band range, with stronger clustering observed in the 7–15 score band (Fig. 2a) than in the 28–42 score band (Fig. 2b).
3.4.2 UAS7TD Versus UAS7OD2AVG
Among the 501 patients with complete data, 406 (81.0%) patients were grouped in the same band for UAS7TD versus UAS7OD2AVG (electronic supplementary Table S2). Similar to the previous analysis of 95 (19.0%) patients with different score band assignments based on UAS7TD versus UAS7OD2AVG, the majority (93/95 [97.9%]) were classified in adjacent score bands (electronic supplementary Table S2). Of all 501 patients, the most commonly observed mismatch between UAS7TD and UAS7OD2AVG disease activity bands was observed in 51 (10.2%) patients classified based on UAS7TD in the 28–42 band, but on UAS7OD2AVG in the 16–27 band. Similar to the scatterplots for UAS7TD versus UAS7OD1MAX, Fig. 3 shows that the UAS7TD and UAS7OD2AVG values for these patients are clustered toward the threshold values of the two score bands.
The coefficient of agreement [κ (95% confidence interval)] was 0.78 (0.75–0.82) for UAS7TD versus UAS7OD1MAX, and 0.82 (0.78–0.85) for UAS7TD versus UAS7OD2AVG, demonstrating substantial to almost perfect agreement between the scores [18].
4 Discussion
This study shows that weekly UAS-based measurements of disease activity in CSU by twice-daily or once-daily assessments by the same patients over the same time period yielded very similar results. We used well-known established methods to analyze the concordance of the scores and their correlation with other PRO measures and found a high positive correlation among the three UAS7 versions analyzed (UAS7TD, UAS7OD1MAX and UAS7OD2AVG), indicating that they provide similar results for the same patient. This analysis was conducted for the first time and the results represent a unique contribution to a better understanding of the instruments used to assess disease activity in CSU, along with their performance. These findings provide evidence to support the use of either of the UAS versions (once-daily/twice-daily assessment) when evaluating CSU activity, as long as the same version is used over time for the same patients within a specific study or clinical practice.
While the descriptive statistics and cross-table analyses show that the majority of patients were assigned to the same disease activity bands of the different scores, some minor discrepancies between score band classifications were observed, which may be due to the differences between the instructions and scoring of the once-daily and twice-daily questionnaires. In the absence of clear guidance for the once-daily version, patients might not be consistent in how they assess and report itch severity over 24 h, and whether they consider average itch or maximum itch when rating their itch severity once daily. Similarly, as the number of hives can fluctuate over 24 h, patients might not be consistent in how they count their hives when they need to report a single total hive count. This is an example that reiterates the need for specific and detailed guidance when developing a PRO instrument; however, as long as the same instrument is used over time in a specific study, the same methodology will apply and scoring should be consistent over time. The high values of weighted kappa coefficients suggest that despite a minority of patient assignments to non-matching score bands between versions, the score bands from the two once-daily scoring methods (UAS7OD1MAX and UAS7OD2AVG) have a high level of overall agreement with the score bands of the UAS7TD.
In addition, UAS7OD2AVG showed higher overall agreement with UAS7TD across analyses than UAS7OD1MAX. This could also be driven by the fact that itch severity is assessed as the average over 24 h in both the UAS7OD2AVG and UAS7TD, whereas in the UAS7OD1MAX it is assessed as the maximum over 24 h. The results of twice-daily itch scores show a difference between morning and evening reports, which confirms the fluctuation over 24 h. Currently, no validation paper or guidance exists for recommending which is a more accurate way of capturing itch severity among CSU patients for the once-daily UAS version. More validation work is required for the once-daily assessment and a manual can be helpful for consistently evaluating itch severity in future studies.
5 Limitations
The current study used descriptive analyses to demonstrate the extent of correlation and level of agreement across the three UAS7 versions and other PRO measures. Multivariable analyses showing how each unit change in each UAS7 version might lead to a corresponding increase/decrease in other scores can be helpful in quantifying the relationship across scores. All analyses were conducted for patients who had non-missing UAS7 values. A higher percentage of patients completed the twice-daily UAS7 version (98.5%) than the once-daily version (82.1%), with the difference being driven by a comparatively low response to the once-daily 24-h exact count of hives question compared with the twice-daily hive ratings question. This could lead to unobserved confounding and might impact the study estimates. In order to facilitate the comparison of twice-daily versus once-daily UAS administrations, the average and maximum score values for the 12-h itch severity scores were used as the 24-h values. An additional limitation for this study is that we do not know how patients quantify itch over the 24-h period when responding to the once-daily version, or even how they quantify it over the 12-h period when answering the twice-daily version. It is known that urticaria symptoms vary from day to day, or even hour to hour [19]. The results of the UAS diary in this study also show that many patients reported different itch scores in the morning and evening, which indicates some variation over a 24-h period. Neither the once-daily nor twice-daily versions of the UAS clearly instruct the patient to consider the overall maximum or average itch severity over the assessment period. Qualitative research could be considered to investigate this for future applications and could provide additional support for this study’s quantitative results.
6 Conclusions
The study found that UAS7 values based on twice-daily versus once-daily evaluation of itch severity and number of hives are highly consistent and correlated. These findings provide evidence to support the use of either version of the weekly UAS when evaluating CSU activity, as long as the chosen version is used consistently over time.
References
Zuberbier T, Aberer W, Asero R, Bindslev-Jensen C, Brzoza Z, Canonica GW, et al. The EAACI/GA(2) LEN/EDF/WAO Guideline for the definition, classification, diagnosis, and management of urticaria: the 2013 revision and update. Allergy. 2014;69(7):868–87.
Maurer M, Magerl M, Metz M, Zuberbier T. Revisions to the international guidelines on the diagnosis and therapy of chronic urticaria. J Dtsch Dermatol Ges. 2013;11(10):971–7 (quiz 8).
Maurer M, Weller K, Bindslev-Jensen C, Gimenez-Arnau A, Bousquet PJ, Bousquet J, et al. Unmet clinical needs in chronic spontaneous urticaria. A GA(2)LEN task force report. Allergy. 2011;66(3):317–30.
O’donnell B, Lawlor F, Simpson J, Morgan M, Greaves M. The impact of chronic urticaria on the quality of life. Br J Dermatol. 1997;136(2):197–201.
Kang MJ, Kim HS, Kim HO, Park YM. The impact of chronic idiopathic urticaria on quality of life in korean patients. Ann Dermatol. 2009;21(3):226–9.
EuroQol Group. EuroQol—a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208.
Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210–6.
Baiardini I, Pasquali M, Braido F, Fumagalli F, Guerra L, Compalati E, et al. A new tool to evaluate the impact of chronic urticaria on quality of life: Chronic Urticaria Quality of Life Questionnaire (CU-QoL). Allergy. 2005;60(8):1073–8.
Snyder CF, Aaronson NK. Use of patient-reported outcomes in clinical practice. Lancet. 2009;374(9687):369–70.
Dobrozsi S, Panepinto J. Patient-reported outcomes in clinical practice. Hematol Am Soc Hematol Educ Program. 2015;2015:501–6.
Mlynek A, Zalewska-Janowska A, Martus P, Staubach P, Zuberbier T, Maurer M. How to assess disease activity in patients with chronic urticaria? Allergy. 2008;63(6):777–80.
Mathias SD, Dreskin SC, Kaplan A, Saini SS, Spector S, Rosen KE. Development of a daily diary for patients with chronic idiopathic urticaria. Ann Allergy Asthma Immunol. 2010;105(2):142–8.
Mathias SD, Crosby RD, Zazzali JL, Maurer M, Saini SS. Evaluating the minimally important difference of the urticaria activity score and other measures of disease activity in patients with chronic idiopathic urticaria. Ann Allergy Asthma Immunol. 2012;108(1):20–4.
US FDA. Guidance for industry. Patient-reported outcome measures: use in medical product development to support labeling claims. US Department of Health and Human Services. Silver Spring, MD: Office of Communications, Division of Drug information, Center for Drug Evaluation and Research; 2009.
Weller K, Maurer M, Grattan C, Nakonechna A, Abuzakouk M, Berard F, et al. ASSURE-CSU: a real-world study of burden of disease in patients with symptomatic chronic spontaneous urticaria. Clin Transl Allergy. 2015;5:29.
Maurer M, Abuzakouk M, Berard F, Canonica W, Oude Elberink H, Gimenez-Arnau A, et al. The burden of chronic spontaneous urticaria is substantial: real-world evidence from ASSURE-CSU. Allergy. 2017;72(12):2005–16.
Stull D, McBride D, Tian H, Gimenez Arnau A, Maurer M, Marsland A, et al. Analysis of disease activity categories in chronic spontaneous/idiopathic urticaria. Br J Dermatol. 2017;177(4):1093–1101.
Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–74.
Flood EM, Zazzali JL, Devlen J. Demonstrating measurement equivalence of the electronic and paper formats of the Urticaria Patient Daily Diary in patients with chronic idiopathic urticaria. Patient. 2013;6(3):225–31.
Acknowledgements
The authors would like to thank Satabdi Chatterjee (Novartis Hyderabad) for her assistance in developing this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
KH, DMcB, LMcL, SH are employed by RTI Health Solutions and as salaried employees did not receive direct compensation from the sponsor. CP was employed by RTI Health Solutions as a salaried employee at the time of the study. In their salaried positions, they work(ed) with a variety of companies and organizations and do not receive direct payments from any company or organization. RTI Health Solutions is a research unit of RTI International, a US not-for profit research Institute, and received consultancy fees from Novartis Pharma AG for this work. RTI Health Solutions and Novartis were involved in the analysis and interpretation of data and the decision to submit these data for publication. MMB and SK are employees of Novartis Pharma AG, who funded the study and the manuscript. HT is an employee of Novartis Pharmaceuticals Corporation, and owns stock from Novartis Pharmaceuticals Corporation. MM is, or recently was, a speaker and/or advisor, and/or received research funding from Novartis.
Funding
This study was funded by Novartis Pharma AG, Basel, Switzerland.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Hollis, K., Proctor, C., McBride, D. et al. Comparison of Urticaria Activity Score Over 7 Days (UAS7) Values Obtained from Once-Daily and Twice-Daily Versions: Results from the ASSURE-CSU Study. Am J Clin Dermatol 19, 267–274 (2018). https://doi.org/10.1007/s40257-017-0331-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40257-017-0331-8