Abstract
Diabetic foot ulcer (DFU) is one of the most severe complications of diabetes mellitus, often resulting in a limb amputation. A cell-based therapy is a highly promising approach for an effective DFU treatment. However, there is no consensus regarding the most effective cell type for DFU treatment. Various cell types contribute to chronic wound healing via different mechanisms. For example, application of keratinocytes can stimulate migration of native keratinocytes from the wound edge, while mesenchymal stem cells can correct limb ischemia. To assess the effectiveness of a certain cell type, it should be administered as a monotherapy without other substances and procedures that have additional therapeutic effects. In the present review, we described therapeutic effects of various cells and provided an overview of clinical studies in which stem and somatic cell-based therapy was administered as a monotherapy. Topical application of somatic cells contributes to DFU healing only, while injection of mesenchymal stem cells and mononuclear cells can break a pathophysiological chain leading from insufficient blood supply to DFU development. At the same time, the systemic use of mesenchymal stem cells carries greater risks. Undoubtedly, cell therapy is a potent tool for the treatment of DFU. However, it is vital to conduct further high-quality clinical research to determine the most effective cell type, dosage and way of administration for DFU treatment.
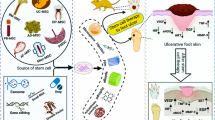
Graphical abstract
Ischemia, neuropathy and neuro-ischemia are underlying factors of diabetic foot ulcer. Stem and somatic cells monotherapy can improve chronic wound healing via different mechanisms.


Similar content being viewed by others
Data Availability
Not applicable.
Code Availability
Not applicable.
Abbreviations
- AD-MSCs:
-
Adipose tissue-derived mesenchymal stem cells
- bFGF:
-
Basic fibroblast growth factor
- BM-MNCs:
-
Bone marrow-derived mononuclear cells
- DFU:
-
Diabetic foot ulcer
- ECM:
-
Extracellular matrix
- EGF:
-
Epidermal growth factor
- EPC:
-
Endothelial progenitor cells
- HGF:
-
Hepatocyte growth factor
- IDF:
-
International Diabetes Federation
- IGF:
-
Insulin growth factor
- Il:
-
Interleukin
- MSCs:
-
Mesenchymal stem cells
- G-CSF:
-
Granulocyte colony-stimulating factor
- MMP:
-
Matrix metalloproteinase
- PB-MNCs:
-
Peripheral blood mononuclear cells
- PDGF:
-
Platelet-derived growth factor
- TGF:
-
Transforming growth factor
- VEGF:
-
Vascular endothelial growth factor
References
IDF Atlas-10th edition. 2021. Available at www.diabetesatlas.org. Accessed 12 Apr 2022.
Zhang, P., Lu, J., Jing, Y., Tang, S., Zhu, D., & Bi, Y. (2017). Global epidemiology of diabetic foot ulceration: A systematic review and meta-analysis. Annals of medicine, 49(2), 106–116.
Apelqvist, J., Bakker, K., Van Houtum, W. H., Schaper, N. C., International Working Group on the Diabetic Foot (IWGDF) Editorial Board. (2008). Practical guidelines on the management and prevention of the diabetic foot: based upon the International Consensus on the Diabetic Foot (2007) Prepared by the International Working Group on the Diabetic Foot. Diabetes/Metabolism research and reviews, 24, S181–S187.
Edmonds, M., Manu, C., & Vas, P. (2021). The current burden of diabetic foot disease. Journal of Clinical Orthopaedics and Trauma, 17, 88–93.
Vuorisalo, S., Venermo, M., & Lepäntalo, M. (2009). Treatment of diabetic foot ulcers. Journal of Cardiovascular Surgery, 50(3), 275.
Yotsu, R. R., Pham, N. M., Oe, M., Nagase, T., Sanada, H., Hara, H., ... & Tamaki, T. (2014). Comparison of characteristics and healing course of diabetic foot ulcers by etiological classification: neuropathic, ischemic, and neuro-ischemic type. Journal of Diabetes and its Complications, 28(4), 528–535.
Mavrogenis, A. F., Megaloikonomos, P. D., Antoniadou, T., Igoumenou, V. G., Panagopoulos, G. N., Dimopoulos, L., ... & Lazaris, A. (2018). Current concepts for the evaluation and management of diabetic foot ulcers. EFORT open reviews, 3(9), 513–525.
Everett, E., & Mathioudakis, N. (2018). Update on management of diabetic foot ulcers. Annals of the New York Academy of Sciences, 1411(1), 153–165.
Meloni, M., Izzo, V., Da Ros, V., Morosetti, D., Stefanini, M., Brocco, E., ... & Uccioli, L. (2020). Characteristics and Outcome for Persons with Diabetic Foot Ulcer and No-Option Critical Limb Ischemia. Journal of Clinical Medicine, 9(11), 3745.
Procházka, V., Gumulec, J., Jalůvka, F., Šalounová, D., Jonszta, T., Czerný, D., ... & Klement, G. L. (2010). Cell therapy, a new standard in management of chronic critical limb ischemia and foot ulcer. Cell transplantation, 19(11), 1413–1424.
Guo, J., Dardik, A., Fang, K., Huang, R., & Gu, Y. (2017). Meta-analysis on the treatment of diabetic foot ulcers with autologous stem cells. Stem Cell Research & Therapy, 8(1), 1–8.
Stessuk, T., Ribeiro‐Paes, J. T., Colpas, P. T., Martins Alves, P. C., Rehder, J., Bosnardo, C. A. F., ... & Puzzi, M. B. (2020). A topical cell therapy approach for diabetic chronic ulcers: Effects of mesenchymal stromal cells associated with platelet‐rich plasma. Journal of Cosmetic Dermatology, 19(10), 2669–2678.
Ravari, H., Hamidi-Almadari, D., Salimifar, M., Bonakdaran, S., Parizadeh, M. R., & Koliakos, G. (2011). Treatment of non-healing wounds with autologous bone marrow cells, platelets, fibrin glue and collagen matrix. Cytotherapy, 13(6), 705–711.
Han, S. K., Choi, K. J., & Kim, W. K. (2004). Clinical application of fresh fibroblast allografts for the treatment of diabetic foot ulcers: A pilot study. Plastic and Reconstructive Surgery, 114(7), 1783–1789.
Han, S. K., Kim, H. S., & Kim, W. K. (2009). Efficacy and safety of fresh fibroblast allografts in the treatment of diabetic foot ulcers. Dermatologic Surgery, 35(9), 1342–1348.
You, H. J., Han, S. K., & Rhie, J. W. (2014). Randomised controlled clinical trial for autologous fibroblast-hyaluronic acid complex in treating diabetic foot ulcers. Journal of Wound Care, 23(11), 521–530.
Hashemi, S. S., Mohammadi, A. A., Kabiri, H., Hashempoor, M. R., Mahmoodi, M., Amini, M., & Mehrabani, D. (2019). The healing effect of Wharton’s jelly stem cells seeded on biological scaffold in chronic skin ulcers: A randomized clinical trial. Journal of Cosmetic Dermatology, 18(6), 1961–1967.
Qin, H. L., Zhu, X. H., Zhang, B., Zhou, L., & Wang, W. Y. (2016). Clinical evaluation of human umbilical cord mesenchymal stem cell transplantation after angioplasty for diabetic foot. Experimental and Clinical Endocrinology & Diabetes, 124(08), 497–503.
Jiang, X., Zhang, H., & Teng, M. (2016). Effectiveness of autologous stem cell therapy for the treatment of lower extremity ulcers: a systematic review and meta-analysis. Medicine, 95(11), e2716.
Falanga, V. (2005). Wound healing and its impairment in the diabetic foot. The Lancet, 366(9498), 1736–1743.
Cañedo-Dorantes, L., & Cañedo-Ayala, M. (2019). Skin acute wound healing: a comprehensive review. International Journal of Inflammation, 2019, 1–5.
McDaniel, J. C., Roy, S., & Wilgus, T. A. (2013). Neutrophil activity in chronic venous leg ulcers—a target for therapy? Wound Repair and Regeneration, 21(3), 339–351.
Lan, C. C. E., Wu, C. S., Huang, S. M., Wu, I. H., & Chen, G. S. (2013). High-glucose environment enhanced oxidative stress and increased interleukin-8 secretion from keratinocytes: New insights into impaired diabetic wound healing. Diabetes, 62(7), 2530–2538.
Ardi, V. C., Kupriyanova, T. A., Deryugina, E. I., & Quigley, J. P. (2007). Human neutrophils uniquely release TIMP-free MMP-9 to provide a potent catalytic stimulator of angiogenesis. Proceedings of the National Academy of Sciences, 104(51), 20262–20267.
Dinh, T., Tecilazich, F., Kafanas, A., Doupis, J., Gnardellis, C., Leal, E., ... & Veves, A. (2012). Mechanisms involved in the development and healing of diabetic foot ulceration. Diabetes, 61(11), 2937–2947.
Acosta, J. B., Garcia del Barco, D., Cibrian Vera, D., Savigne, W., Lopez-Saura, P., Guillen Nieto, G., & Schultz, G. S. (2008). The pro-inflammatory environment in recalcitrant diabetic foot wounds. International Wound Journal, 5(4), 530–539.
Liu, Y., Min, D., Bolton, T., Nubé, V., Twigg, S. M., Yue, D. K., & McLennan, S. V. (2009). Increased matrix metalloproteinase-9 predicts poor wound healing in diabetic foot ulcers: response to Muller et al. Diabetes Care, 32(11), e137–e137.
Hariono, M., Yuliani, S. H., Istyastono, E. P., Riswanto, F. D., & Adhipandito, C. F. (2018). Matrix metalloproteinase 9 (MMP9) in wound healing of diabetic foot ulcer: Molecular target and structure-based drug design. Wound Medicine, 22, 1–13.
Li, G., Zou, X., Zhu, Y., Zhang, J., Zhou, L., Wang, D., ... & Chen, Z. (2017). Expression and influence of matrix metalloproteinase–9/tissue inhibitor of metalloproteinase–1 and vascular endothelial growth factor in diabetic foot ulcers. The international Journal of Lower Extremity Wounds, 16(1), 6–13.
Uccioli, L., Izzo, V., Meloni, M., Vainieri, E., Ruotolo, V., & Giurato, L. (2015). Non-healing foot ulcers in diabetic patients: General and local interfering conditions and management options with advanced wound dressings. Journal of Wound Care, 24(Sup4b), 35–42.
Huang, S. M., Wu, C. S., Chiu, M. H., Wu, C. H., Chang, Y. T., Chen, G. S., & Lan, C. C. E. (2019). High glucose environment induces M1 macrophage polarization that impairs keratinocyte migration via TNF-α: An important mechanism to delay the diabetic wound healing. Journal of Dermatological Science, 96(3), 159–167.
Mirza, R., & Koh, T. J. (2011). Dysregulation of monocyte/macrophage phenotype in wounds of diabetic mice. Cytokine, 56(2), 256–264.
Goren, I., Müller, E., Schiefelbein, D., Gutwein, P., Seitz, O., Pfeilschifter, J., & Frank, S. (2009). Akt1 controls insulin-driven VEGF biosynthesis from keratinocytes: Implications for normal and diabetes-impaired skin repair in mice. Journal of Investigative Dermatology, 129(3), 752–764.
Peplow, P. V., & Baxter, G. D. (2012). Gene expression and release of growth factors during delayed wound healing: A review of studies in diabetic animals and possible combined laser phototherapy and growth factor treatment to enhance healing. Photomedicine and Laser Surgery, 30(11), 617–636.
Doxey, D. L., Ng, M. C., Dill, R. E., & Iacopino, A. M. (1995). Platelet-derived growth factor levels in wounds of diabetic rats. Life Sciences, 57(11), 1111–1123.
Brown, D. L., Kane, C. D., Chernausek, S. D., & Greenhalgh, D. G. (1997). Differential expression and localization of insulin-like growth factors I and II in cutaneous wounds of diabetic and nondiabetic mice. The American Journal of Pathology, 151(3), 715.
Bitar, M. S., & Labbad, Z. N. (1996). Transforming growth factor-β and insulin-like growth factor-I in relation to diabetes-induced impairment of wound healing. Journal of Surgical Research, 61(1), 113–119.
Loots, M. A., Lamme, E. N., Mekkes, J. R., Bos, J. D., & Middelkoop, E. (1999). Cultured fibroblasts from chronic diabetic wounds on the lower extremity (non-insulin-dependent diabetes mellitus) show disturbed proliferation. Archives of Dermatological Research, 291(2), 93–99.
Januszyk, M., Chen, K., Henn, D., Foster, D. S., Borrelli, M. R., Bonham, C. A., ... & Gurtner, G. C. (2020). Characterization of diabetic and non-diabetic foot ulcers using single-cell RNA-sequencing. Micromachines, 11(9), 815.
Loots, M. A., Kenter, S. B., Au, F. L., Van Galen, W. J. M., Middelkoop, E., Bos, J. D., & Mekkes, J. R. (2002). Fibroblasts derived from chronic diabetic ulcers differ in their response to stimulation with EGF, IGF-I, bFGF and PDGF-AB compared to controls. European Journal of Cell Biology, 81(3), 153–160.
Brem, H., Stojadinovic, O., Diegelmann, R. F., Entero, H., Lee, B., Pastar, I., ... & Tomic-Canic, M. (2007). Molecular markers in patients with chronic wounds to guide surgical debridement. Molecular Medicine, 13(1), 30-39.
Shao, H., Li, Y., Pastar, I., Xiao, M., Prokupets, R., Liu, S., ... & Liu, Z. J. (2020). Notch1 signaling determines the plasticity and function of fibroblasts in diabetic wounds. Life Science Alliance, 3(12), e202000769.
Stojadinovic, O., Brem, H., Vouthounis, C., Lee, B., Fallon, J., Stallcup, M., ... & Tomic-Canic, M. (2005). Molecular pathogenesis of chronic wounds: the role of β-catenin and c-myc in the inhibition of epithelialization and wound healing. The American Journal of Pathology, 167(1), 59-69.
Uzun, E., Güney, A., Gönen, Z. B., Özkul, Y., Kafadar, İH., Günay, M., & Mutlu, M. (2021). Intralesional allogeneic adipose-derived stem cells application in chronic diabetic foot ulcer: Phase I/2 safety study. Foot and Ankle Surgery, 27(6), 636–642.
Suzdaltseva, Y., Zhidkih, S., Kiselev, S. L., & Stupin, V. (2020). Locally delivered umbilical cord mesenchymal stromal cells reduce chronic inflammation in long-term nonhealing wounds: a randomized study. Stem Cells International, 2020, 1–11.
Wang, J., Zeng, X. X., Cai, W., Han, Z. B., Zhu, L. Y., Liu, J. Y., & Xu, J. X. (2021). Safety and efficacy of placenta-derived mesenchymal stem cell treatment for diabetic patients with critical limb ischemia: A pilot study. Experimental and Clinical Endocrinology & Diabetes, 129(07), 542–548.
Maksimova, N., Krasheninnikov, M., Zhang, Y., Ponomarev, E., Pomytkin, I., Melnichenko, G., & Lyundup, A. (2017). Early passage autologous mesenchymal stromal cells accelerate diabetic wound re-epithelialization: A clinical case study. Cytotherapy, 19(12), 1548–1550.
Zeng, X., Tang, Y., Hu, K., Jiao, W., Ying, L., Zhu, L., ... & Xu, J. (2017). Three-week topical treatment with placenta-derived mesenchymal stem cells hydrogel in a patient with diabetic foot ulcer: a case report. Medicine, 96(51), e9212.
Wu, S. C., Pollak, R., Frykberg, R. G., Zhou, W., Karnoub, M., Jankovic, V., ... & Chitkara, D. (2017). Safety and efficacy of intramuscular human placenta‐derived mesenchymal stromal‐like cells (cenplacel [PDA‐002]) in patients who have a diabetic foot ulcer with peripheral arterial disease. International Wound Journal, 14(5), 823–829.
Li, X. Y., Zheng, Z. H., Li, X. Y., Guo, J., Zhang, Y., Li, H., ... & Wu, Z. B. (2013). Treatment of foot disease in patients with type 2 diabetes mellitus using human umbilical cord blood mesenchymal stem cells: response and correction of immunological anomalies. Current Pharmaceutical Design, 19(27), 4893–4899.
Lee, H. C., An, S. G., Lee, H. W., Park, J. S., Cha, K. S., Hong, T. J., ... & Jung, J. S. (2012). Safety and effect of adipose tissue-derived stem cell implantation in patients with critical limb ischemia: a pilot study. Circulation Journal, 76(7), 1750–1760.
Lu, D., Chen, B., Liang, Z., Deng, W., Jiang, Y., Li, S., ... & Chen, S. (2011). Comparison of bone marrow mesenchymal stem cells with bone marrow-derived mononuclear cells for treatment of diabetic critical limb ischemia and foot ulcer: a double-blind, randomized, controlled trial. Diabetes Research and Clinical Practice, 92(1), 26–36.
Dash, N. R., Dash, S. N., Routray, P., Mohapatra, S., & Mohapatra, P. C. (2009). Targeting nonhealing ulcers of lower extremity in human through autologous bone marrow-derived mesenchymal stem cells. Rejuvenation Research, 12(5), 359–366.
Kirana, S., Stratmann, B., Prante, C., Prohaska, W., Koerperich, H., Lammers, D., ... & Tschoepe, D. (2012). Autologous stem cell therapy in the treatment of limb ischaemia induced chronic tissue ulcers of diabetic foot patients. International Journal of Clinical Practice, 66(4), 384–393.
Subrammaniyan, R., Amalorpavanathan, J., Shankar, R., Rajkumar, M., Baskar, S., Manjunath, S. R., ... & Abraham, S. (2011). Application of autologous bone marrow mononuclear cells in six patients with advanced chronic critical limb ischemia as a result of diabetes: our experience. Cytotherapy, 13(8), 993-999.
Franz, R. W., Parks, A., Shah, K. J., Hankins, T., Hartman, J. F., & Wright, M. L. (2009). Use of autologous bone marrow mononuclear cell implantation therapy as a limb salvage procedure in patients with severe peripheral arterial disease. Journal of Vascular Surgery, 50(6), 1378–1390.
Kirana, S., Stratmann, B., Lammers, D., Negrean, M., Stirban, A., Minartz, P., ... & Tschoepe, D. (2007). Wound therapy with autologous bone marrow stem cells in diabetic patients with ischaemia‐induced tissue ulcers affecting the lower limbs. International Journal of Clinical Practice, 61(4), 690–694.
Humpert, P. M., Bärtsch, U., Konrade, I., Hammes, H. P., Morcos, M., Kasper, M., ... & Nawroth, P. P. (2005). Locally applied mononuclear bone marrow cells restore angiogenesis and promote wound healing in a type 2 diabetic patient. Experimental and Clinical Endocrinology & Diabetes, 113(09), 538–540.
Huang, P., Li, S., Han, M., Xiao, Z., Yang, R., & Han, Z. C. (2005). Autologous transplantation of granulocyte colony–stimulating factor–mobilized peripheral blood mononuclear cells improves critical limb ischemia in diabetes. Diabetes Care, 28(9), 2155–2160.
Mohammadzadeh, L., Samedanifard, S. H., Keshavarzi, A., Alimoghaddam, K., Larijani, B., Ghavamzadeh, A., ... & Mohajeri-Tehrani, M. R. (2013). Therapeutic outcomes of transplanting autologous granulocyte colony-stimulating factor-mobilised peripheral mononuclear cells in diabetic patients with critical limb ischaemia. Experimental and Clinical Endocrinology & Diabetes, 121(01), 48–53.
Tanaka, R., Masuda, H., Kato, S., Imagawa, K., Kanabuchi, K., Nakashioya, C., ... & Miyasaka, M. (2014). Autologous G-CSF-mobilized peripheral blood CD34+ cell therapy for diabetic patients with chronic nonhealing ulcer. Cell Transplantation, 23(2), 167–179.
Nilforoushzadeh, M., Jaffary, F., Siavash, M., Ansari, N., Siadat, A., & Heidari, A. (2016). Autologous fibroblast suspension for the treatment of refractory diabetic foot ulcer. Indian Journal of Dermatology, Venereology, and Leprology, 82(1), 105–105.
Marston, W. A., Hanft, J., Norwood, P., Pollak, R., Dermagraft Diabetic Foot Ulcer Study Group. (2003). The efficacy and safety of Dermagraft in improving the healing of chronic diabetic foot ulcers: results of a prospective randomized trial. Diabetes Care, 26(6), 1701–1705.
Hwang, Y. G., Lee, J. W., Park, K. H., & Han, S. H. (2019). Allogeneic keratinocyte for intractable chronic diabetic foot ulcers: A prospective observational study. International Wound Journal, 16(2), 486–491.
You, H. J., Han, S. K., Lee, J. W., & Chang, H. (2012). Treatment of diabetic foot ulcers using cultured allogeneic keratinocytes—a pilot study. Wound Repair and Regeneration, 20(4), 491–499.
Moustafa, M., Bullock, A. J., Creagh, F. M., Heller, S., Jeffcoate, W., Game, F., ... & MacNeil, S. (2007). Randomized, controlled, single-blind study on use of autologous keratinocytes on a transfer dressing to treat nonhealing diabetic ulcers. Regenerative Medicine, 2(6), 887–902.
Bayram, Y., Deveci, M., Imirzalioglu, N., Soysal, Y., & Sengezer, M. (2005). The cell based dressing with living allogenic keratinocytes in the treatment of foot ulcers: A case study. British Journal of Plastic Surgery, 58(7), 988–996.
Moustafa, M., Simpson, C., Glover, M., Dawson, R. A., Tesfaye, S., Creagh, F. M., ... & MacNeil, S. (2004). A new autologous keratinocyte dressing treatment for non‐healing diabetic neuropathic foot ulcers. Diabetic Medicine, 21(7), 786-789.
Liu, L., Yu, Y., Hou, Y., Chai, J., Duan, H., Chu, W., ... & Du, J. (2014). Human umbilical cord mesenchymal stem cells transplantation promotes cutaneous wound healing of severe burned rats. PloS one, 9(2), e88348.
Li, H., Ziemer, M., Stojanovic, I., Saksida, T., Maksimovic-Ivanic, D., Mijatovic, S., ... & Savkovic, V. (2022). Mesenchymal Stem Cells From Mouse Hair Follicles Reduce Hypertrophic Scarring in a Murine Wound Healing Model. Stem Cell Reviews and Reports, 1–17.
Ahn, S. Y., Maeng, Y. S., Kim, Y. R., Choe, Y. H., Hwang, H. S., & Hyun, Y. M. (2020). In vivo monitoring of dynamic interaction between neutrophil and human umbilical cord blood-derived mesenchymal stem cell in mouse liver during sepsis. Stem Cell Research & Therapy, 11(1), 1–15.
Maggini, J., Mirkin, G., Bognanni, I., Holmberg, J., Piazzón, I. M., Nepomnaschy, I., ... & Geffner, J. R. (2010). Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like profile. PloS one, 5(2), e9252.
Xu, J., Zgheib, C., Hodges, M. M., Caskey, R. C., Hu, J., & Liechty, K. W. (2017). Mesenchymal stem cells correct impaired diabetic wound healing by decreasing ECM proteolysis. Physiological Genomics, 49(10), 541–548.
Zhang, N., Li, J., Luo, R., Jiang, J., & Wang, J. A. (2008). Bone marrow mesenchymal stem cells induce angiogenesis and attenuate the remodeling of diabetic cardiomyopathy. Experimental and Clinical Endocrinology & Diabetes, 116(02), 104–111.
Ge, Q., Zhang, H., Hou, J., Wan, L., Cheng, W., Wang, X., ... & Wu, X. (2018). VEGF secreted by mesenchymal stem cells mediates the differentiation of endothelial progenitor cells into endothelial cells via paracrine mechanisms. Molecular Medicine Reports, 17(1), 1667–1675.
Huang, C., Luo, W., Wang, Q., Ye, Y., Fan, J., Lin, L., ... & Tang, Y. (2021). Human mesenchymal stem cells promote ischemic repairment and angiogenesis of diabetic foot through exosome miRNA-21–5p. Stem Cell Research, 52, 102235.
Sasaki, M., Abe, R., Fujita, Y., Ando, S., Inokuma, D., & Shimizu, H. (2008). Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. The Journal of Immunology, 180(4), 2581–2587.
Javazon, E. H., Keswani, S. G., Badillo, A. T., Crombleholme, T. M., Zoltick, P. W., Radu, A. P., ... & Flake, A. W. (2007). Enhanced epithelial gap closure and increased angiogenesis in wounds of diabetic mice treated with adult murine bone marrow stromal progenitor cells. Wound Repair and Regeneration, 15(3), 350–359.
Li, M., Zhao, Y., Hao, H., Dai, H., Han, Q., Tong, C., ... & Fu, X. (2015). Mesenchymal stem cell–conditioned medium improves the proliferation and migration of keratinocytes in a diabetes-like microenvironment. The International Journal of Lower Extremity Wounds, 14(1), 73–86.
Shabbir, A., Cox, A., Rodriguez-Menocal, L., Salgado, M., & Badiavas, E. V. (2015). Mesenchymal stem cell exosomes induce proliferation and migration of normal and chronic wound fibroblasts, and enhance angiogenesis in vitro. Stem Cells and Development, 24(14), 1635–1647.
Saheli, M., Bayat, M., Ganji, R., Hendudari, F., Kheirjou, R., Pakzad, M., ... & Piryaei, A. (2020). Human mesenchymal stem cells-conditioned medium improves diabetic wound healing mainly through modulating fibroblast behaviors. Archives of Dermatological Research, 312(5), 325–336.
Du, W. J., Chi, Y., Yang, Z. X., Li, Z. J., Cui, J. J., Song, B. Q., ... & Han, Z. C. (2016). Heterogeneity of proangiogenic features in mesenchymal stem cells derived from bone marrow, adipose tissue, umbilical cord, and placenta. Stem Cell Research & Therapy, 7(1), 1–11.
Hsiao, S. T. F., Asgari, A., Lokmic, Z., Sinclair, R., Dusting, G. J., Lim, S. Y., & Dilley, R. J. (2012). Comparative analysis of paracrine factor expression in human adult mesenchymal stem cells derived from bone marrow, adipose, and dermal tissue. Stem Cells and Development, 21(12), 2189–2203.
Pomatto, M., Gai, C., Negro, F., Cedrino, M., Grange, C., Ceccotti, E., ... & Camussi, G. (2021). Differential therapeutic effect of extracellular vesicles derived by bone marrow and adipose mesenchymal stem cells on wound healing of diabetic ulcers and correlation to their cargoes. International Journal of Molecular Sciences, 22(8), 3851.
Kim, H., Han, J. W., Lee, J. Y., Choi, Y. J., Sohn, Y. D., Song, M., & Yoon, Y. S. (2015). Diabetic mesenchymal stem cells are ineffective for improving limb ischemia due to their impaired angiogenic capability. Cell Transplantation, 24(8), 1571–1584.
Megallaa, M. H., Ismail, A. A., Zeitoun, M. H., & Khalifa, M. S. (2019). Association of diabetic foot ulcers with chronic vascular diabetic complications in patients with type 2 diabetes. Diabetes & Metabolic Syndrome: Clinical Research & Reviews, 13(2), 1287–1292.
Madanchi, N., Tabatabaei-Malazy, O., Pajouhi, M., Heshmat, R., Larijani, B., & Mohajeri-Tehrani, M. R. (2013). Who are diabetic foot patients? A descriptive study on 873 patients. Journal of Diabetes & Metabolic Disorders, 12(1), 1–6.
Volmer-Thole, M., & Lobmann, R. (2016). Neuropathy and diabetic foot syndrome. International Journal of Molecular Sciences, 17(6), 917.
Cuende, N., Rico, L., & Herrera, C. (2012). Concise review: Bone marrow mononuclear cells for the treatment of ischemic syndromes: Medicinal product or cell transplantation? Stem Cells Translational Medicine, 1(5), 403–408.
Tateishi-Yuyama, E., Matsubara, H., Murohara, T., Ikeda, U., Shintani, S., Masaki, H., ... & Therapeutic Angiogenesis using Cell Transplantation (TACT) Study Investigators. (2002). Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. The Lancet, 360(9331), 427–435.
Durdu, S., Akar, A. R., Arat, M., Sancak, T., Eren, N. T., & Ozyurda, U. (2006). Autologous bone-marrow mononuclear cell implantation for patients with Rutherford grade II-III thromboangiitis obliterans. Journal of Vascular Surgery, 44(4), 732–739.
Ornellas, F. M., Ornellas, D. S., Martini, S. V., Castiglione, R. C., Ventura, G. M., Rocco, P. R., ... & Morales, M. M. (2017). Bone marrow-derived mononuclear cell therapy accelerates renal ischemia-reperfusion injury recovery by modulating inflammatory, antioxidant and apoptotic related molecules. Cellular Physiology and Biochemistry, 41(5), 1736–1752.
Umemura, Y., Ogura, H., Matsuura, H., Ebihara, T., Shimizu, K., & Shimazu, T. (2018). Bone marrow-derived mononuclear cell therapy can attenuate systemic inflammation in rat heatstroke. Scandinavian Journal of Trauma, Resuscitation and Emergency Medicine, 26(1), 1–9.
Holler, V., Buard, V., Roque, T., Squiban, C., Benderitter, M., Flamant, S., & Tamarat, R. (2019). Early and late protective effect of bone marrow mononuclear cell transplantation on radiation-induced vascular dysfunction and skin lesions. Cell Transplantation, 28(1), 116–128.
Kamihata, H., Matsubara, H., Nishiue, T., Fujiyama, S., Tsutsumi, Y., Ozono, R., ... & Iwasaka, T. (2001). Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation, 104(9), 1046–1052.
Cui, K., Wang, M., Yu, L., Ren, X., Cui, H., Yu, X. F., ... & Wang, J. (2016). Transplantation of autologous bone marrow mononuclear cells regulates inflammation in a rabbit model of carotid artery atherosclerosis. Journal of Vascular Research, 53(3–4), 196–205.
Mildner, M., Hacker, S., Haider, T., Gschwandtner, M., Werba, G., Barresi, C., ... & Ankersmit, H. J. (2013). Secretome of peripheral blood mononuclear cells enhances wound healing. PloS one, 8(3), e60103.
Huang, P. P., Yang, X. F., Li, S. Z., Wen, J. C., Zhang, Y., & Han, Z. C. (2007). Randomised comparison of G-CSF-mobilized peripheral blood mononuclear cells versus bone marrow-mononuclear cells for the treatment of patients with lower limb arteriosclerosis obliterans. Thrombosis and Haemostasis, 98(12), 1335–1342.
Capiod, J. C., Tournois, C., Vitry, F., Sevestre, M. A., Daliphard, S., Reix, T., ... & Pignon, B. (2009). Characterization and comparison of bone marrow and peripheral blood mononuclear cells used for cellular therapy in critical leg ischaemia: towards a new cellular product. Vox Sanguinis, 96(3), 256–265.
Jamaludin, W. F. W., Yusoff, F. M., & DipNurs, N. A. (2018). Autologous mononuclear cells from different sources are seen to improve wound healing in patients with haematological malignancies. The Malaysian Journal of Pathology, 40(1), 61–67.
Yang, J., Ii, M., Kamei, N., Alev, C., Kwon, S. M., Kawamoto, A., ... & Asahara, T. (2011). CD34+ cells represent highly functional endothelial progenitor cells in murine bone marrow. PloS one, 6(5), e20219.
Kudo, F. A., Nishibe, T., Nishibe, M., & Yasuda, K. (2003). Autologous transplantation of peripheral blood endothelial progenitor cells (CD34^ sup+^) for therapeutic angiogenesis in patients with critical limb ischemia. International Angiology, 22(4), 344.
Kawamoto, A., Katayama, M., Handa, N., Kinoshita, M., Takano, H., Horii, M., ... & Asahara, T. (2009). Intramuscular transplantation of G‐CSF‐mobilized CD34+ cells in patients with critical limb ischemia: a phase I/IIa, multicenter, single‐blinded, dose‐escalation clinical trial. Stem Cells, 27(11), 2857–2864.
Kinoshita, M., Fujita, Y., Katayama, M., Baba, R., Shibakawa, M., Yoshikawa, K., ... & Kawamoto, A. (2012). Long-term clinical outcome after intramuscular transplantation of granulocyte colony stimulating factor-mobilized CD34 positive cells in patients with critical limb ischemia. Atherosclerosis, 224(2), 440–445.
Fadini, G. P., & Avogaro, A. (2013). Diabetes impairs mobilization of stem cells for the treatment of cardiovascular disease: a meta-regression analysis. International Journal of Cardiology, 168(2), 892–897.
Li, P. J., Jin, T., Luo, D. H., Shen, T., Mai, D. M., Hu, W. H., & Mo, H. Y. (2015). Effect of prolonged radiotherapy treatment time on survival outcomes after intensity-modulated radiation therapy in nasopharyngeal carcinoma. PloS one, 10(10), e0141332.
Tanaka, R., Masuda, H., Fujimura, S., Ito-Hirano, R., Arita, K., Kakinuma, Y., ... & Asahara, T. (2018). Quality-quantity control culture enhances vasculogenesis and wound healing efficacy of human diabetic peripheral blood CD34+ cells. Stem Cells Translational Medicine, 7(5), 428–438.
Dubský, M., Jirkovska, A., Bem, R., Nemcova, A., Fejfarova, V., & Jude, E. B. (2017). Cell therapy of critical limb ischemia in diabetic patients–State of art. Diabetes Research and Clinical Practice, 126, 263–271.
Stanley, A., & Osler, T. (2001). Senescence and the healing rates of venous ulcers. Journal of Vascular Surgery, 33(6), 1206–1211.
Mansbridge, J. N., Liu, K., Pinney, R. E., Patch, R., Ratcliffe, A., & Naughton, G. K. (1999). Growth factors secreted by fibroblasts: Role in healing diabetic foot ulcers. Diabetes, Obesity and Metabolism, 1(5), 265–279.
Pinney, E., Liu, K., Sheeman, B., & Mansbridge, J. (2000). Human three-dimensional fibroblast cultures express angiogenic activity. Journal of Cellular Physiology, 183(1), 74–82.
Ferrer, R. A., Saalbach, A., Grünwedel, M., Lohmann, N., Forstreuter, I., Saupe, S., ... & Franz, S. (2017). Dermal fibroblasts promote alternative macrophage activation improving impaired wound healing. Journal of Investigative Dermatology, 137(4), 941–950.
Krejci-Papa, N. C., Hoang, An., & Hansbrough, J. F. (1999). Fibroblast sheets enable epithelialization of wounds that do not support keratinocyte migration. Tissue Engineering, 5(6), 555–561.
Driskell, R. R., Lichtenberger, B. M., Hoste, E., Kretzschmar, K., Simons, B. D., Charalambous, M., ... & Watt, F. M. (2013). Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature, 504(7479), 277–281.
Janson, D. G., Saintigny, G., Van Adrichem, A., Mahé, C., & El Ghalbzouri, A. (2012). Different gene expression patterns in human papillary and reticular fibroblasts. Journal of Investigative Dermatology, 132(11), 2565–2572.
Brown, L. F., Yeo, K., Berse, B., Yeo, T. K., Senger, D. R., Dvorak, H. F., & Van De Water, L. (1992). Expression of vascular permeability factor (vascular endothelial growth factor) by epidermal keratinocytes during wound healing. Journal of Experimental Medicine, 176(5), 1375–1379.
Halaban, R., Langdon, R., Birchall, N., Cuono, C., Baird, A., Scott, G., ... & McGuire, J. (1988). Basic fibroblast growth factor from human keratinocytes is a natural mitogen for melanocytes. The Journal of Cell Biology, 107(4), 1611–1619.
Ansel, J. C., Tiesman, J. P., Olerud, J. E., Krueger, J. G., Krane, J. F., Tara, D. C., ... & Hart, C. E. (1993). Human keratinocytes are a major source of cutaneous platelet-derived growth factor. The Journal of Clinical Investigation, 92(2), 671–678.
Haynes, S. L., Shuttleworth, C. A., & Kielty, C. M. (1997). Keratinocytes express fibrillin and assemble microfibrils: Implications for dermal matrix organization. British Journal of Dermatology, 137(1), 17–23.
Velander, P., Theopold, C., Bleiziffer, O., Bergmann, J., Svensson, H., Feng, Y., & Eriksson, E. (2009). Cell suspensions of autologous keratinocytes or autologous fibroblasts accelerate the healing of full thickness skin wounds in a diabetic porcine wound healing model. Journal of Surgical Research, 157(1), 14–20.
Brain, A., Purkis, P., Coates, P., Hackett, M., Navsaria, H., & Leigh, I. (1989). Survival of cultured allogeneic keratinocytes transplanted to deep dermal bed assessed with probe specific for Y chromosome. British Medical Journal, 298(6678), 917–919.
Beele, H., Naeyaert, J. M., Goeteyn, M., De Mil, M., & Kint, A. (1991). Repeated cultured epidermal allografts in the treatment of chronic leg ulcers of various origins. Dermatology, 183(1), 31–35.
Krasilnikova, O. A., Klabukov, I. D., Baranovskii, D. S., Shegay, P. V., & Kaprin, A. D. (2021). The new legal framework for minimally manipulated cells expands the possibilities for cell therapy in Russia. Cytotherapy, 23(8), 754–755.
Svensjö, T., Yao, F., Pomahac, B., & Eriksson, E. (2001). Autologous keratinocyte suspensions accelerate epidermal wound healing in pigs. Journal of Surgical Research, 99(2), 211–221.
Jang, H., Kim, Y. H., Kim, M. K., Lee, K. H., & Jeon, S. (2013). Wound-healing potential of cultured epidermal sheets is unaltered after lyophilization: a preclinical study in comparison to cryopreserved CES. BioMed Research International, 2013, 907209.
Sakamoto, M., Ogino, S., Shimizu, Y., Inoie, M., Lee, S., Yamanaka, H., ... & Morimoto, N. (2020). Human cultured epidermis accelerates wound healing regardless of its viability in a diabetic mouse model. Plos one, 15(8), e0237985.
Maksimova N. V., Michenko A. V., Krasilnikova O. A., Klabukov I. D., Gadaev I. Y., Krasheninnikov M. E., ... & Lyundup A. V. (2022). Mesenchymal stromal cell therapy alone does not lead to complete restoration of skin parameters in diabetic foot patients within a 3-year follow-up period. BioImpacts, 12(1), 51–55.
Author information
Authors and Affiliations
Contributions
OK and IK designed the study. DB contributed to the interpretation of the data. OK, IK, and DB drafted the manuscript. AL, PS and AK supervised the study. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflicts of Interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to the Topical Collection: Special Issue on Stem Cell Technology and Skin Disorders (Dermatology): from Stem Cell Biology to Clinical Application
Guest Editor: Ali Golchin
Rights and permissions
About this article
Cite this article
Krasilnikova, O.A., Baranovskii, D.S., Lyundup, A.V. et al. Stem and Somatic Cell Monotherapy for the Treatment of Diabetic Foot Ulcers: Review of Clinical Studies and Mechanisms of Action. Stem Cell Rev and Rep 18, 1974–1985 (2022). https://doi.org/10.1007/s12015-022-10379-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-022-10379-z