Abstract
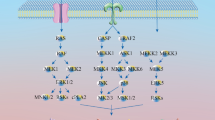
Cytoskeletal reorganization, including reconstruction of actin fibers and microtubules, is essential for various biological processes, such as cell migration, proliferation and dendrite formation. We show here that methylophiopogonanone B (MOPB) induces cell morphological change via melanocyte dendrite retraction and stress fiber formation. Since members of the Rho family of small GTP-binding proteins act as master regulators of dendrite formation and actin cytoskeletal reorganization, and activated Rho promotes dendrite retraction and stress fiber formation, we studied the effects of MOPB on the small GTPases using normal human epidermal melanocytes and HeLa cells. In in vitro binding assay, MOPB significantly increased GTP-Rho, but not GTP-Rac or GTP-CDC42. Furthermore, a Rho inhibitor, a Rho kinase inhibitor and a small GTPase inhibitor each blocked MOPB-induced stress fiber formation. The effect of MOPB on actin reorganization was blocked in a Rho dominant negative mutant. These results suggest MOPB acts via the Rho signaling pathway, and it may directly or indirectly activate Rho. Quantitative Western blot analysis indicated that MOPB also induced microtubule destabilization and tubulin depolymerization. Thus, MOPB appears to induce Rho activation, resulting in actin cytoskeletal reorganization, including dendrite retraction and stress fiber formation.
Similar content being viewed by others
Abbreviations
- MOPB:
-
methylophiopogonanone B
- NHEM:
-
normal human epidermal melanocytes
- ROCK:
-
Rho kinase
References
Ridley AJ, Hall A (1992) The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 70: 389–399
Etienne-Manneville S, Hall A (2002) Rho GTPases in cell biology. Nature 420: 629–635
Wherlock M, Mellor H (2002) The Rho GTPase family: a Racs to Wrchs story. J Cell Sci 115: 239–240
Burridge K, Wennerberg K (2004) Rho and Rac take center stage. Cell 116: 167–179
Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A (1992) The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell 70: 401–410
Jalink K, van Corven EJ, Hengeveld T, Morii N, Narumiya S, Moolenaar WH (1994) Inhibition of lysophosphatidate- and thrombin-induced neurite retraction and neuronal cell rounding by ADP ribosylation of the small GTP-binding protein Rho. J Cell Biol 126: 801–810
Ridley AJ (1994) Signal transduction through the GTP-binding proteins Rac and Rho. J Cell Sci Suppl 18: 127–131
Kozma R, Ahmed S, Best A, Lim L (1995) The Ras-related protein Cdc42Hs and bradykinin promote formation of peripheral actin microspikes and filopodia in Swiss 3T3 fibroblasts. Mol Cell Biol 15: 1942–1952
Scott G (2002) Rac and Rho: the story behind melanocyte dendrite formation. Pigment Cell Res 15: 322–330
Nakazawa K, Damour O, Collombel C (1993) Modulation of normal human melanocyte dendricity by growth-promoting agents. Pigment Cell Res 6: 406–416
Duval C, Regnier M, Schmidt R (2001) Distinct melanogenic response of human melanocytes in mono-culture, in co-culture with keratinocytes and in reconstructed epidermis, to UV exposure. Pigment Cell Res 14: 348–355
Virador V, Matsunaga N, Matsunaga J, Valencia J, Oldham RJ, Kameyama K, Peck GL, Ferrans VJ, Vieira WD, Abdel-Malek ZA, Hearing VJ (2001) Production of melanocyte-specific antibodies to human melanosomal proteins: expression patterns in normal human skin and in cutaneous pigment lesions. Pigment Cell Res 14: 289–297
Scott G, Cassidy L (1998) Rac1 mediates dendrite formation in response to melanocyte stimulating hormone and ultraviolet light in a murine melanoma model. J Invest Dermatol 111: 243–250
Yasui H, Katoh H, Yamaguchi Y, Aoki J, Fujita H, Mori K, Negishi M: Differential responses to nerve growth factor and epidermal growth factor in neurite outgrowth of PC12 cells are determined by Rac1 activation systems. J Biol Chem 276: 15298–15305, 2001
Busca R, Bertolotto C, Abbe P, Englaro W, Ishizaki T, Narumiya S, Boquet P, Ortonne JP, Ballotti R (1998) Inhibition of Rho is required for cAMP-induced melanoma cell differentiation. Mol Biol Cell 9: 1367–1378
Katoh H, Aoki J, Ichikawa A, Negishi M (1998) p160 RhoA-binding kinase ROKalpha induces neurite retraction. J Biol Chem 273: 2489–2492
Sander EE, ten Klooster JP, van Delft S, van der Kammen RA, Collard JG (1999) Rac downregulates Rho activity: reciprocal balance between both GTPases determines cellular morphology and migratory behavior. J Cell Biol 147: 1009–1022
Threadgill R, Bobb K, Ghosh A (1997) Regulation of dendritic growth and remodeling by Rho, Rac, and Cdc42. Neuron 19: 625–634
Lacour JP, Gordon PR, Eller M, Bhawan J, Gilchrest BA (1992) Cytoskeletal events underlying dendrite formation by cultured pigment cells. J Cell Physiol 151: 287–299
Enomoto T (1996) Microtubule disruption induces the formation of actin stress fibers and focal adhesions in cultured cells: possible involvement of the rho signal cascade. Cell Struct Funct 21: 317–326
Beutler JA, Hamel E, Vlietinck AJ, Haemers A, Rajan P, Roitman JN, Cardellina JH 2nd, Boyd MR (1998) Structure-activity requirements for flavone cytotoxicity and binding to tubulin. J Med Chem 41: 2333–2338
Kim JH, Sohn SH, Hong SW (2001) Three-dimensional quantitative structure activity relationship studies on the flavone cytotoxicity and binding to tubulin. J Photosci 8: 119–121
Tada A, Kanamaru A, Ito Y (2005) Control of melanosome transfer by promoting shrinkage or expansion of melanocyte dendrites. IFSCC Mag 8: 293–299
Ito Y, Kanamaru A, Tada A (2006) Centaureidin promotes dendrite retraction of melanocytes by activating Rho. Biochim Biophys Acta 1760: 487–494
Ito Y, Kanamaru A, Tada A: Effects of methylophiopogonanone B on melanosome transfer and dendrite retraction. J Dermatol Sci 42: 68–70, 2006
Tada A, Kasai R, Saitoh T, Shoji J (1980) Studies on the constituents of Ophiopogonis Tuber. V. Isolation of a novel class of homoisoflavonoids and determination of their structures. Chem Pharm Bull 28: 1477–1484
Amano M, Mukai H, Ono Y, Chihara K, Matsui T, Hamajima Y, Okawa K, Iwamatsu A, Kaibuchi K: Identification of a putative target for Rho as the serine–threonine kinase protein kinase N. Science 271: 648–650, 1996
Kuroda S, Fukata M, Kobayashi K, Nakafuku M, Nomura N, Iwamatsu A, Kaibuchi K (1996) Identification of IQGAP as a putative target for the small GTPases, Cdc42 and Rac1. J Biol Chem 271: 23363–23367
Kuroda S, Fukata M, Fujii K, Nakamura T, Izawa I, Kaibuchi K (1997) Regulation of cell–cell adhesion of MDCK cells by Cdc42 and Rac1 small GTPases. Biochem Biophys Res Commun 240: 430–435
Zhou J, Panda D, Landen JW, Wilson L, Joshi HC (2002) Minor alteration of microtubule dynamics causes loss of tension across kinetochore pairs and activates the spindle checkpoint. J Biol Chem 277: 17200–17208
Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K (1996) Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science 273: 245–248
Danowski BA (1989) Fibroblast contractility and actin organization are stimulated by microtubule inhibitors. J Cell Sci 93: 255–266
Anderson S, DiCesare L, Tan I, Leung T, SundarRaj N (2004) Rho-mediated assembly of stress fibers is differentially regulated in corneal fibroblasts and myofibroblasts. Exp Cell Res 298: 574–583
Wadkins RM, Jares-Erijman EA, Klement R, Rudiger A, Jovin TM (1996) Actinomycin D binding to single-stranded DNA: sequence specificity and hemi-intercalation model from fluorescence and 1H NMR spectroscopy. J Mol Biol 262: 53–68
Kominek LA (1975) Cycloheximide production by Streptomyces griseus: alleviation of end-product inhibition by dialysis-extraction fermentation. Antimicrob Agents Chemother 7: 861–863
Wilson L, Panda D, Jordan MA (1999) Modulation of microtubule dynamics by drugs: a paradigm for the actions of cellular regulators. Cell Struct Funct 24: 329–335
Islam MN, Iskander MN (2004) Microtubulin binding sites as target for developing anticancer agents. Mini Rev Med Chem 4: 1077–1104
Zhou J, Giannakakou P (2005) Targeting microtubules for cancer chemotherapy. Curr Med Chem Anti-Canc Agents 5: 65–71
Acknowledgments
We thank Drs. S. Kuroda (Tokyo University) and K. Kaibuchi (Nagoya University) for providing small GTPase plasmids. We also thank Drs. K. Mizuno and K. Ohashi (Tohoku University) for helpful discussions, and T. Katagiri and Dr. Y. Fujiki for encouragement and suggestions concerning all aspects of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ito, Y., Kanamaru, A. & Tada, A. A novel agent, methylophiopogonanone B, promotes Rho activation and tubulin depolymerization. Mol Cell Biochem 297, 121–129 (2007). https://doi.org/10.1007/s11010-006-9336-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-006-9336-y