Abstract
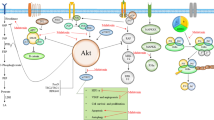
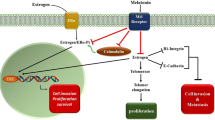
This review article discusses recent work on the melatonin-mediated circadian regulation and integration of molecular and metabolic signaling mechanisms involved in human breast cancer growth and the associated consequences of circadian disruption by exposure to light-at-night (LAN). The anti-proliferative effects of the circadian melatonin signal are, in general, mediated through mechanisms involving the activation of MT1 melatonin receptors expressed in human breast cancer cell lines and xenografts. In estrogen receptor-positive (ERα+) human breast cancer cells, melatonin suppresses both ERα mRNA expression and estrogen-induced transcriptional activity of the ERα via MT1-induced activation of Gαi2 signaling and reduction of cAMP levels. Melatonin also regulates the transcriptional activity of additional members of the nuclear receptor super-family, enzymes involved in estrogen metabolism, and the expression of core clock and clock-related genes. The anti-invasive/anti-metastatic actions of melatonin involve the blockade of p38 phosphorylation and matrix metalloproteinase expression. Melatonin also inhibits the growth of human breast cancer xenografts via MT1-mediated suppression of cAMP leading to a blockade of linoleic acid (LA) uptake and its metabolism to the mitogenic signaling molecule 13-hydroxyoctadecadienoic acid (13-HODE). Down-regulation of 13-HODE reduces the activation of growth factor pathways supporting cell proliferation and survival. Finally, studies in both rats and humans indicate that light-at-night (LAN) induced circadian disruption of the nocturnal melatonin signal activates human breast cancer growth, metabolism, and signaling, providing the strongest mechanistic support, thus far, for epidemiological studies demonstrating the elevated breast cancer risk in night shift workers and other individuals increasingly exposed to LAN.




Similar content being viewed by others
Abbreviations
- LAN:
-
light at night
- ERα:
-
estrogen receptor alpha
- SCN:
-
suprachiasmatic nucleus
- NR:
-
nuclear receptor
- 13-HODE:
-
13-hydroxyoctadecadienoic acid
References
Brainard GC, Hanifin JP, Greeson JM, et al. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J Neurosci. 2001;21:6405–12.
Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–3.
Hastings M, Reddy AB, Maywood ES. A clockwork web: circadian timing in brain and periphery, in health and disease. Nat Rev Neurosci. 2003;4:649–61.
Claustrat B, Brun J, Chazot G. The basic physiology and pathophysiology of melatonin. Sleep Med Rev. 2005;9:11–24.
Reiter RJ. Melatonin: the chemical expression of darkness. Mol Cell Endocrinol. 1991;79:C153–8.
Stevens RG, Blask DE, Brainard GC, et al. Meeting report: the role of environmental lighting and circadian disruption in cancer and other diseases. Environ Health Perspect. 2007;115:1357–62.
Blask DE. Melatonin, sleep disturbance and cancer risk. Sleep Med Rev. 2009;13:257–64.
You S, Wood PA, Xiong Y, et al. Daily coordination of cancer growth and circadian clock gene expression. Breast Cancer Res Treat. 2005;91:47–60.
Kohsaka A, Bass J. A sense of time: how molecular clocks organize metabolism. Trends Endocrinol Metab. 2007;18:4–11.
Blask DE, Dauchy RT, Sauer LA. Putting cancer to sleep at night: the neuroendocrine/circadian melatonin signal. Endocrine. 2005;27:179–88.
Hill SM, Blask DE. Effects of the pineal hormone melatonin on the proliferation and morphological characteristics of human breast cancer cells (MCF-7) in culture. Cancer Res. 1988;48:6121–6.
Blask DE, Sauer LA, Dauchy RT. Melatonin as a chronobiotic/anticancer agent: cellular, biochemical, and molecular mechanisms of action and their implications for circadian-based cancer therapy. Curr Top Med Chem. 2002;2:113–32.
Cos S, Blask DE, Lemus-Wilson A, et al. Effects of melatonin on the cell cycle kinetics and “estrogen-rescue” of MCF-7 human breast cancer cells in culture. J Pineal Res. 1991;10:36–42.
Hill SM, Frasch T, Xiang S, et al. Molecular mechanisms of melatonin anticancer effects. Integr Cancer Ther. 2009;8:337–46.
Blask DE, Brainard GC, Dauchy RT, et al. Melatonin-depleted blood from premenopausal women exposed to light at night stimulates growth of human breast cancer xenografts in nude rats. Cancer Res. 2005;65:11174–84.
Brydon L, Roka F, Petit L, et al. Dual signaling of human Mel1a melatonin receptors via G(i2), G(i3), and G(q/11) proteins. Mol Endocrinol. 1999;13:2025–38.
Lai L, Yuan L, Chen Q, et al. The Galphai and Galphaq proteins mediate the effects of melatonin on steroid/thyroid hormone receptor transcriptional activity and breast cancer cell proliferation. J Pineal Res. 2008;45:476–88.
Collins A, Yuan L, Kiefer TL, et al. Overexpression of the MT1 melatonin receptor in MCF-7 human breast cancer cells inhibits mammary tumor formation in nude mice. Cancer Lett. 2003;189:49–57.
Yuan L, Collins AR, Dai J, et al. MT(1) melatonin receptor overexpression enhances the growth suppressive effect of melatonin in human breast cancer cells. Mol Cell Endocrinol. 2002;192:147–56.
Lai L, Yuan L, Cheng Q, et al. Alteration of the MT1 melatonin receptor gene and its expression in primary human breast tumors and breast cancer cell lines. Breast Cancer Res Treat. 2009;118:293–305.
Molis TM, Spriggs LL, Hill SM. Modulation of estrogen receptor mRNA expression by melatonin in MCF-7 human breast cancer cells. Mol Endocrinol. 1994;8:1681–90.
Ram PT, Kiefer T, Silverman M, et al. Estrogen receptor transactivation in MCF-7 breast cancer cells by melatonin and growth factors. Mol Cell Endocrinol. 1998;141:53–64.
Hill SM, Frasch T, Xiang S, Yuan L, et al. Molecular mechanisms of melatonin anticancer effects. Integr Cancer Res. 2009;8:337–46.
Del Rio B, Garcia Pedrero JM, Martinez-Campa C, et al. Melatonin, an endogenous-specific inhibitor of estrogen receptor alpha via calmodulin. J Biol Chem. 2004;279:38294–302.
Dai J, Inscho EW, Yuan L, et al. Modulation of intracellular calcium and calmodulin by melatonin in MCF-7 human breast cancer cells. J Pineal Res. 2002;32:112–9.
Kiefer TL, Lai L, Yuan L, et al. Differential regulation of estrogen receptor alpha, glucocorticoid receptor and retinoic acid receptor alpha transcriptional activity by melatonin is mediated via different G proteins. J Pineal Res. 2005;38:231–9.
Dai J, Ram PT, Yuan L, et al. Transcriptional repression of RORalpha activity in human breast cancer cells by melatonin. Mol Cell Endocrinol. 2001;176:111–20.
Lapin V. Pineal gland and malignancy. Osterr Z Onkol. 1976;3:51–60.
Blask DE. The pineal: an oncostatic gland? In: Rj R, editor. The pineal gland. New York: Raven; 1984. p. 253–84.
Cos S, Fernandez R, Guezmes A, et al. Influence of melatonin on invasive and metastatic properties of MCF-7 human breast cancer cells. Cancer Res. 1998;58:4383–90.
Leon-Blanco MM, Guerrero JM, Reiter RJ, et al. Melatonin inhibits telomerase activity in the MCF-7 tumor cell line both in vivo and in vitro. J Pineal Res. 2003;35:204–11.
Tan M, Yao J, Yu D. Overexpression of the c-erbB-2 gene enhanced intrinsic metastasis potential in human breast cancer cells without increasing their transformation abilities. Cancer Res. 1997;57:1199–205.
Schmid BC, Rudas M, Rezniczek GA, et al. CXCR4 is expressed in ductal carcinoma in situ of the breast and in atypical ductal hyperplasia. Breast Cancer Res Treat. 2004;84:247–50.
Mao L, Slakey LM, Jones FE, Burow ME, Hill SM. Inhibition of breast cancer cell invasion by melatonin is mediated through regulation of p38 mitogen-activated protien kinase signaling pathway. Breast Cancer Res. 2010;12:R107 Epub.
Kim MS, Lee EJ, Kim HR, et al. p38 kinase is a key signaling molecule for H-Ras-induced cell motility and invasive phenotype in human breast epithelial cells. Cancer Res. 2003;63:5454–61.
Sauer LA, Dauchy RT, Blask DE. Polyunsaturated fatty acids, melatonin, and cancer prevention. Biochem Pharmacol. 2001;61:1455–62.
Blask DE, Dauchy RT, Sauer LA et al. Oral melatonin supplementation in rats and a human subject suppresses the growth activity of steroid receptor negative human breast cancer xenografts in female nude rats via an MT1 receptor-mediated suppression of signal tranduction and linoleic acid uptake and metabolism. In: AACR Meeting Abstracts; 2005. pp. 1358.
Reyes N, Reyes I, Tiwari R, et al. Effect of linoleic acid on proliferation and gene expression in the breast cancer cell line T47D. Cancer Lett. 2004;209:25–35.
Blask DE, Sauer LA, Dauchy RT, et al. Melatonin inhibition of cancer growth in vivo involves suppression of tumor fatty acid metabolism via melatonin receptor-mediated signal transduction events. Cancer Res. 1999;59:4693–701.
Wu J, Dauchy RT, Tirrell PC, et al. Light at night activates IGF-R/PDK signaling and accelerates tumor growth in human breast cancer xenografts. Cancer Res. 2011;71:2622–31.
Stevens RG. Electric power use and breast cancer: a hypothesis. Am J Epidemiol. 1987;125:556–61.
Stevens R, London SJ. Breast cancer. In: Stevens RG, Wilson BW, Anderson LE, editors. The melatonin hypothesis: breast cancer and the use of electric power. Columbus: Battelle Press; 1997. p. 9–24.
Stevens RG, Rea MS. Light in the built environment: potential role of circadian disruption in endocrine disruption and breast cancer. Cancer Causes Control. 2001;12:279–87.
Stevens RG. Light-at-night, circadian disruption and breast cancer: assessment of existing evidence. Int J Epidemiol. 2009;38:963–70.
Hansen J. Increased breast cancer risk among women who work predominantly at night. Epidemiology. 2001;12:74–7.
Davis S, Mirick DK, Stevens RG. Night shift work, light at night, and risk of breast cancer. J Natl Cancer Inst. 2001;93:1557–62.
Schernhammer ES, Laden F, Speizer FE, et al. Rotating night shifts and risk of breast cancer in women participating in the nurses’ health study. J Natl Cancer Inst. 2001;93:1563–8.
Straif K, Baan R, Grosse Y, et al. Carcinogenicity of shift-work, painting, and fire-fighting. Lancet Oncol. 2007;8:1065–6.
Schernhammer ES, Hankinson SE. Urinary melatonin levels and breast cancer risk. J Natl Cancer Inst. 2005;97:1084–7.
Kloog I, Stevens RG, Haim A et al. Nighttime light level co-distributes with breast cancer incidence worldwide. Cancer Causes Control. 2010;21(12):2059–68.
Schernhammer ES, Berrino F, Krogh V, et al. Urinary 6-sulfatoxymelatonin levels and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 2008;100:898–905.
Dauchy RT, Sauer LA, Blask DE, et al. Light contamination during the dark phase in “photoperiodically controlled” animal rooms: effect on tumor growth and metabolism in rats. Lab Anim Sci. 1997;47:511–8.
Dauchy RT, Blask DE, Sauer LA, et al. Dim light during darkness stimulates tumor progression by enhancing tumor fatty acid uptake and metabolism. Cancer Lett. 1999;144:131–6.
Cailotto C, Lei J, Van Der Vliet J, et al. Effects of nocturnal light on (clock) gene expression in peripheral organs: a role for the autonomic innervation of the liver. PLoS One. 2009;4:e5650.
Kennaway DJ, Owens JA, Voultsios A, et al. Metabolic homeostasis in mice with disrupted Clock gene expression in peripheral tissues. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1528–37.
Guillaumond F, Dardente H, Giguere V, et al. Differential control of Bmal1 circadian transcription by REV-ERB and ROR nuclear receptors. J Biol Rhythms. 2005;20:391–403.
Xiang S, Coffelt SB, Mao L, et al. Period-2: a tumor suppressor gene in breast cancer. J Circadian Rhythms. 2008;6:4–12.
Yang X, Wood PA, Du-Quiton J, Ansell CM, Hrushesky WJ. Down reguation of circadian clock gene Period 2 accelerates breast cancer growth by alteringit daily growth rhythm. Breast Cancer Res Treat. 2009;117:423–31.
Kim E, Yoo Y, Yang W, Lim Y, Na T, Lee I, et al. Transcriptiohnal activation of Hif1 by RORα and its role in hypoxia signaling. Arterioscler Thromb Vasc Biol. 2008;28:1796–802.
Xiang S, Mao L, Yuan L, Hill SM. Effect of melatonin on the clock genes in breast epithelial and breast cancer cells. 100th Annual Mtg. American Association for Cancer Research, Denver, CO., Abst. #3596; 2009.
Fu L, Pelicano H, Liu J, et al. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell. 2002;111:41–50.
Nakahata Y, Kaluzova M, Grimaldi B, et al. The NAD+ −dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell. 2008;134:329–40.
Liu T, Liu PY, Marshall GM. The critical role of the class III histone deacetylase SIRT1 in cancer. Cancer Res. 2009;69:1702–5.
Hardeland R, Reiter RJ, Poeggeler B, et al. The significance of the metabolism of the neurohormone melatonin: antioxidative protection and formation of bioactive substances. Neurosci Biobehav Rev. 1993;17:347–57.
Poeggeler B, Thuermann S, Dose A, et al. Melatonin’s unique radical scavenging properties – roles of its functional substituents as revealed by a comparison with its structural analogs. J Pineal Res. 2002;33:20–30.
Matuszak Z, Rezka K, Chignell CF. Reaction of melatonin and related indoles with hydroxyl radicals: EPR and spin trapping investigations. Free Rad Biol Med. 1997;23:367–72.
Stasica P, Ulanski P, Rosiak JM. Melatonin as a hydroxyl radical scavenger. J Pineal Res. 1998;25:65–6.
Uz T, Manev H. Circadian expression of pineal 5-lipoxygenase mRNA. Neuroreport. 1998;9:783–6.
Zhang H, Akbar M, Kim HY. Melatonin: an endogenous negative modulator of 12-lipoxygenatyion in the rat pineal gland. Biochem J. 1999;344:487–93.
Hardeland R. Melatonin: multiple functions in signaling and protection. In: Altmeyer P, Hoffman K, Stucker M, editors. Skin Cancer and UV Radiation. Berlin-Heidelberg: Springer; 1997. p. 186–98.
Genova ML, Pich MM, Bernacchia A, et al. The mitochondrial production of reactive oxygen species in relation to aging and pathology. Ann NY Acad Sci. 2004;1011:86–100.
Acknowledgements
The work by the authors presented in this article was generously supported by National Institutes of Health grants 5R01CA054152-18 (SMH), Army Department Of Defense grant DAMD-123201 (SMH), National Institute of Environmental Health Sciences R21ES11659 (DEB), Edwin W. Pauley Foundation (DEB) and Grants for Laboratory Animal Science (GLAS) (RTD).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hill, S.M., Blask, D.E., Xiang, S. et al. Melatonin and Associated Signaling Pathways that Control Normal Breast Epithelium and Breast Cancer. J Mammary Gland Biol Neoplasia 16, 235–245 (2011). https://doi.org/10.1007/s10911-011-9222-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10911-011-9222-4