Abstract
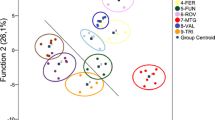
Nestmate recognition plays a key role in kin selection to maintain colony integrity in social insects. Previous studies have demonstrated that nestmate recognition is dependent on detection of cuticular hydrocarbons. However, the absence of intraspecific aggression between some colonies of Isoptera and social Hymenoptera questions whether kin recognition must occur in social insects. The purpose of this study was to determine if cuticular hydrocarbon similarity and high genetic relatedness could explain the lack of intraspecific aggression among and within colonies of the introduced subterranean termite Reticulitermes santonensis. We performed both GC analysis of cuticular hydrocarbons and genotyping by using 10 DNA microsatellite loci on the same 10 workers from each of 14 parisian colonies. Multivariate analyses demonstrated correspondence between cuticular hydrocarbon patterns and genetic variation. By using a redundancy analysis combining chemical and genetic data, we found that a few hydrocarbons (mainly short vs. long chains; saturated vs. unsaturated alkanes) were associated with most genetic variation. We also found a strong positive correlation between chemical and genetic distances between colonies, thus providing evidence of a genetic basis for cuticular hydrocarbon variation. However, genetic distance did not account for all chemical variation, thus suggesting that some hydrocarbon variation was environmentally derived. Investigation at the intracolony level indicated that cuticular hydrocarbons did not depend on colony social structure. Based on our findings, we speculate that the absence of intraspecific aggression in R. santonensis may result from a loss of diversity in genetically derived recognition compounds in this species that presumably descended from R. flavipes populations imported from North America.





Similar content being viewed by others
References
Austin, J. W., Szalanski, A. L., Scheffrahn, R. H., Messenger, M. T., Dronnet, S., and Bagnères A.-G. 2005. Genetic evidence for the synonymy of two Reticulitermes species: Reticulitermes flavipes (Kollar) and Reticulitermes santonensis (Feytaud). Ann. Entomol. Soc. Am. 98:395–401.
Bagnères, A.-G., Clément, J.-C., Blum, M. S., Severson, R. F., Joulie, C., and Lange, C. 1990. Cuticular hydrocarbons and defensive compounds of Reticulitermes flavipes (Kollar) and R. santonensis (Feytaud): Polymorphism and chemotaxonomy. J. Chem. Ecol. 16:3213–3244.
Belkhir, K., Borsa, P., Chikhi, L., Raufaste, N., and Bonhomme, F. 1996–2001. genetix 4.04 Logiciel sous windows TM pour la Génétique des Populations. Laboratoire Génome, Populations, Interactions, CNRS UMR 5000, Université de Montpellier.
Beye, M., Neumann, P., Chapuisat, M., Pamilo, P., and Moritz, R. F. A. 1998. Nestmate recognition and the genetic relatedness of nests in the ant Formica pratensis. Behav. Ecol. Sociobiol. 43:67–72.
Breed, M. D. and Bennett, G. W. 1987. Kin recognition in highly eusocial insects, pp. 243–285, in D. J. C. Fletcher, and C. D. Michener (eds.). Kin Recognition in Animals. Wiley and Sons.
Bulmer, M. S. and Traniello, J. F. A. 2002. Lack of aggression and spatial association of colony members in Reticulitermes flavipes. J. Insect Behav. 15:121–126.
Carlin, N. F. and Hölldobler, B. 1986. The kin recognition system of carpenter ants (Camponotus spp.). I. Hierarchical cues in small colonies. Behav. Ecol. Sociobiol. 19:123–134.
Cavalli-Sforza, L. L. and Edwards, A. W. F. 1967. Phylogenetic analysis: Models and estimation procedures. Am. J. Hum. Genet. 19:233–257.
Clément, J.-L. 1986. Open and closed societies in Reticulitermes termites (Isoptera, Rhinotermitidae): Geographic and seasonal variations. Sociobiology 11:311–323.
Clément, J.-L. and Bagnères, A.-G. 1998. Nestmate recognition in termites, pp. 126–155, in R. K. Vander Meer, M. D. Breed, K. E. Espelie, and M. L. Winston (eds.). Pheromone Communication in Social Insects. Ants, Wasps, Bees, and Termites. Westview Press, Boulder, CO.
Cornelius, M. L. and Osbrink, W. L. 2003. Agonistic interactions between colonies of the Formosan subterranean termite (Isoptera: Rhinotermitidae) in New Orleans, Louisiana. Environ. Entomol. 32:1002–1009.
Dronnet, S., Bagnères, A.-G., Juba, T. R., and Vargo, E. L. 2004. Polymorphic microsatellite loci in the European subterranean termite, Reticulitermes santonensis Feytaud. Mol. Ecol. Notes 4:127–129.
Dronnet, S., Chapuisat, M., Vargo, E. L., Lohou, C., and Bagnères, A.-G. 2005a. Genetic analysis of the breeding system of an invasive subterranean termite, Reticulitermes santonensis, in urban and natural habitats. Mol. Ecol. 14:1311–1320.
Dronnet, S., Simon, X., Verhaeghe, J.-C., Rasmont, P., and Errard, C. 2005b. Bumblebee inquilinism in Bombus (Fernaldaepsithyrus) sylvestris Lepeletier (Hymenoptera, Apidae): Behavioural and chemical analyses of host–parasite interactions. Apidologie 36:59–70.
Felsenstein, J., 1993. Phylogeny Inference Package (PHYLIP), Version 3.5c University of Washington, Seattle.
Fitch, W. M. and Margoliash, E. 1967. Construction of phylogenetic trees. Science 155:279–284.
Florane, C. B., Bland, J. M., Husseneder, C., and Raina, A. K. 2004. Diet-mediated inter-colonial aggression in the Formosan subterranean termite Coptotermes formosanus. J. Chem. Ecol. 30:2559–2574.
Giraud, T., Pedersen, J. S., and Keller, L. 2002. Evolution of supercolonies: The Argentine ants of southern Europe. Proc. Natl. Acad. Sci. USA 99:6075–6079.
Goudet, J. 1995. FSTAT (version 1.2): A computer program to calculate F-statistics. J. Heredity 86:485–486.
Grace, J. K. 1996. Absence of overt agonistic behavior in a northern population of Reticulitermes flavipes (Isoptera: Rhinotermitidae). Sociobiology 28:103–110.
Hamilton, W. D. 1964. The genetic evolution of social behaviour. J. Theor. Biol. 71:1–52.
Haverty, M. I. and Thorne, B. L. 1989. Agonistic behavior correlated with hydrocarbon phenotypes in dampwood termites, Zootermopsis (Isoptera: Termopsidae). J. Insect Behav. 2:523–543.
Howard, R. W. and Blomquist, G. J. 2005. Ecological, behavioral, and biochemical aspects of insect hydrocarbons. Annu. Rev. Entomol. 50:371–393.
Husseneder, C. and Grace, J. K. 2001. Evaluation of DNA fingerprinting, aggression tests, and morphometry as tools for colony delineation of the Formosan subterranean termite. J. Insect Behav. 14:173–186.
Husseneder, C., Kaib, M., Epplen, C., Epplen, J. T., and Brandl, R. 1997. Small-scale population structure of the termite Schedorhinotermes lamanianus: Aggression modulated by genetic and environmental factors. Mitt. Dtsch. Ges. Allg. Angew. Entomol. 11:183–187.
Husseneder, C., Brandl, R., Epplen, C., Epplen, J. T., and Kaib, M. 1998. Variation between and within colonies in the termite: Morphology, genomic DNA, and behaviour. Mol. Ecol. 7:983–990.
Kaib, M., Jmhasly, P., Wilfert, L., Durka, W., Franke, S., Francke, W., Leuthold, R. H., and Brandl, R. 2004. Cuticular hydrocarbons and aggression in the termite Macrotermes subhyalinus. J. Chem. Ecol. 30:365–385.
Le Breton, J., Delabie, J. H. C., Chazeau, J., Dejean, A., and Jourdan, H. 2004. Experimental evidence of large-scale unicoloniality in the tramp ant Wasmannia auropunctata (Roger). J. Insect Behav. 17:263–271.
Legendre, P. and Legendre, L. 1998. Numerical Ecology. 2nd English ed. Elsevier, Amsterdam.
Leponce, M., Roisin, Y., and Pasteels, J. 1996. Intraspecific interactions in a community of arboreal nesting termites (Isoptera: Termitidae). J. Insect Behav. 9:799–817.
Lipkovich, I. and Smith, E. P. 2002. Biplot and Singular Value Decomposition Macros for Excel©. J. Stat. Software 7:1–15.
Matsuura, K. 2001. Nestmate recognition mediated by intestinal bacteria in a termite, Reticulitermes speratus. Oikos 92:20–26.
Nei, M. 1987. Molecular Evolutionary Genetics. Columbia University Press, New York.
Ozaki, M., Wada-Katsumata, A., Fujikawa, K., Iwasaki, M., Yokohari, F., Satoji, Y., Nisimura, T., and Yamaoka, R. 2005. Ant nestmate and non-nestmate discrimination by a chemosensory sensillum. Science 309:311–314.
Pirk, C. W. W., Neumann, P., Moritz, R. F. A., and Pamilo, P. 2001. Intranest relatedness and nestmate recognition in the meadow ant Formica pratensis (R.). Behav. Ecol. Sociobiol. 49:366–374.
Queller, D. C. and Goodnight, K. F. 1989. Estimating relatedness using genetic markers. Evolution 43:258–275.
Raymond, M. and Rousset, F. 1995. GENEPOP (version 1.2): A population genetics software for exact tests and ecumenism. J. Heredity 86:248–249.
Su, N.-Y. and Haverty, M. I. 1991. Agonistic behavior among colonies of the Formosan subterranean termite, Coptotermes formosanus Shiraki (Isoptera: Rhinotermitidae), from Florida and Hawaii: Lack of correlation with cuticular hydrocarbon composition. J. Insect Behav. 4:115–128.
Ter Braak, C. J. F. 1987. Ordination, pp. 29–77, in R. H. G. Jongman, C. J. F. Ter Braak, O. F. R. Van Tongeren (eds.). Data Analysis in Community and Landscape Ecology. Pudoc, Wageningen.
Thorne, B. L., Traniello, J. F. A., Adams, E. S., and Bulmer, M. 1999. Reproductive dynamics and colony structure of subterranean termites of the genus Reticulitermes (Isoptera Rhinotermitidae): A review of the evidence from behavioral, ecological and genetic studies. Ethol. Ecol. Evol. 11:149–169.
Tsutsui, N. D., Suarez, A. V., Holway, D. A., and Case, T. J. 2000. Reduced genetic variation in the success of an invasive species. Proc. Natl. Acad. Sci. USA 97:5948–5953.
Vander Meer, R. K. and Morel, L. 1998. Nestmate recognition in ants, pp. 79–103, in R. K. Vander Meer, M. D. Breed, K. E. Espelie, and M. L. Winston (eds.). Pheromone Communication in Social Insects: Ants, Wasps, Bees and Termites. Westview Press, Boulder, CO.
Vargo, E. L. 2000. Polymorphism at trinucleotide microsatellite loci in the subterranean termite Reticulitermes flavipes. Mol. Ecol. 9:817–829.
Vargo, E. L. 2003. Hierarchical analysis of colony and population genetic structure of the eastern subterranean termite, Reticulitermes flavipes, using two classes of molecular markers. Evolution 57:2805–2818.
Vieau, F. 2001. Comparison of the spatial distribution and reproductive cycle of Reticulitermes santonensis Feytaud and Reticulitermes lucifugus grassei Clément (Isoptera, Rhinotermitidae) suggests that they represent introduced and native species, respectively. Insectes Soc. 48:57–62.
Wilson, E. O. 1971. The Insect Societies. Harvard University Press, Cambridge.
Acknowledgements
We are grateful to the “Section de Lutte contre les termites” (SMASH, Paris) and the pest control operators (HIE Piguy, Hygiène Office, Techmohygiène & APBM Bruant) for kindly providing some samples. We thank P. Legendre (Université de Montréal) for advice on the published material on redundancy analysis, and K. A. Copren, C. Husseneder, M. Kaib, N. D. Tsutsui, and E. L. Vargo for comments on this manuscript, and we thank A. Corsini for help in improving the English of the manuscript. This study was supported by a contract between the Centre de la Recherche Scientifique (CNRS) and the City of Paris (Direction des Parcs, Jardins et Espaces verts).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dronnet, S., Lohou, C., Christides, JP. et al. Cuticular Hydrocarbon Composition Reflects Genetic Relationship Among Colonies of the Introduced Termite Reticulitermes santonensis Feytaud. J Chem Ecol 32, 1027–1042 (2006). https://doi.org/10.1007/s10886-006-9043-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-006-9043-x