Summary
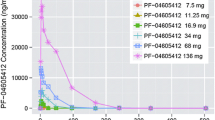
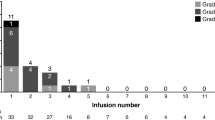
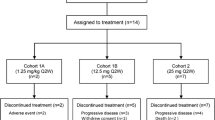
This study assessed the safety, immunogenicity, and pharmacokinetics of etaracizumab, a monoclonal antibody directed against the αvβ3 integrin, in patients with advanced malignancies. Four cohorts of four patients received escalating dose of etaracizumab as a 30-min intravenous infusion, first as a single test dose, followed-up 2–5 weeks later by weekly doses. Sixteen patients with advanced solid tumors received a total of 309 cycles of etaracizumab at doses ranging 1–6 mg/kg. The mean number of weekly infusions was 19 (ranging 5–53). Frequently reported adverse events were grades 1–2 asthenia (15 patients) and infusion reactions (9 patients). At 1 mg/kg, one patient experienced grade 3 chills with the first infusion. Other grade 3 toxicities included reversible hyponatremia, hypophosphatemia and hyponatremia in one patient each at 1, 4 and 6 mg/kg, respectively. No patient experienced treatment delay/discontinuation due to an adverse event. The half-life of etaracizumab ranged 49–180 h with a nonlinear increase in terminal half-life with increasing doses. There was no objective response but five patients experienced a stable disease of >6-month duration. Etaracizumab was well-tolerated at doses up to 6 mg/kg with no evidence of immunogenicity. The safety profile of etaracizumab warrants further exploration in ongoing phase I/II trials.


Similar content being viewed by others
References
Sledge G, Miller K (2002) Angiogenesis and antiangiogenic therapy. Curr Probl Cancer 26:1–60
Liotta LA, Steeg PS, Stetler-Stevenson WG (1991) Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell 64:327–336
Hynes RO (1992) Integrins: versatility, modulation, and signaling in cell adhesion. Cell 69:11–25
Defilippi P, Bozzo C, Volpe G, Romano G, Venturino M, Silengo L, Tarone G (1994) Integrin mediated signal transduction in human endothelial cells: analysis of tryrosine phosphorylation events. Cell Adhes Commun 2:75–86
Bischoff J (1995) Approaches to studying cell adhesion molecules in angiogenesis. Trends Cell Biol 5:69–73
Ruoslahti E (1991) Integrins. J Clin Invest 87:1–5
Leavesley D, Schwartz MA, Rosenfeld M, Ceresh DA (1993) Integrin beta 1- and beta 3-mediated endothelial cell migration is triggered through distinct signaling mechanisms. J Cell Biol 121:163–170
Paszek MJ et al (2005) Tensional homeostasis and the malignant phenotype. Cancer Cell 8:241–254
Sethi T et al (1999) Extracellular matrix proteins protect small cell lung cancer cells against apoptosis: a mechanism for small cell lung cancer growth and drug resistance in vivo. Nat Med 5:662–668
Brooks PC, Clark RA, Cheresh DA (1994) Requirement of vascular integrin αvβ3 for angiogenesis. Science 264:569–571
Felding-Habermann B et al (1992) Involvement of integrin alpha V gene expression in human melanoma tumorigenicity. J Clin Invest 89(6):2018–2022
Van Belle PA et al (1999) Progression-related expression of beta3 integrin in melanomas and naevi. Human Pathol 30(5):562–567
Mitjans F, Meyer T, Fittschen C, Goodman S, Jonczyk A, Marshall JF, Reyes G, Piulats J (2000) In vivo therapy of malignant melanoma by means of antagonists of αV integrins. Int J Cancer 87:716–732
Takano S et al (2000) Tissue factor, osteopontin, alphavbeta3 integrin expression in microvasculature of gliomas associated with vascular endothelial growth factor expression. Br J Cancer 82(12):1967–1973
Chatterjee S et al (2000) Human malignant glioma therapy using anti-alpha(v)beta3 integrin agents. J Neurooncol 46(2):135–144
Montgomery AMP, Reisfeld RA, Cheresh DA (1994) Integrin αvβ3 rescues melanoma cells from apoptosis in three-dimensional dermal collagen. Proc Natl Acad Sci USA 91:8856–8860
Petitclerc E et al (1999) Integrin αvβ3 promotes M21 melanoma growth in human skin by regulating tumor cell survival. Cancer Res 59:2724–2730
Rabb H, Barroso-Vicens E, Adams R, Pow-Sang J, Ramirez G (1996) Alpha-V/beta-3 and alpha-V/beta-5 integrin distribution in neoplastic kidney. Am J Nephrol 16:402–408
Wechsel HW, Petri E, Feil G, Nelde HJ, Bichler KH, Loesr W (1999) Renal cell carcinoma: immunohistological investigation of expression of the integrin alpha v beta 3. Anticancer Res 19:1529–1532
Felding-Habermann B et al (2001) Integrin activation controls metastasis in human breast cancer. Proc Natl Acad Sci USA 98(4):1853–1858
Liapsis H et al (1997) Expression of alpha(v)beta3 integrin is less frequent in ovarian epithelial tumors of low malignant potential in contrast to ovarian carcinomas. Human Pathol 28(4):443–449
Liapsis H, Flath A, Kitazawa S (1996) Integrin alpha V beta 3 expression by bone-residing breast cancer metastases. Diagn Mol Pathol 5(2):127–135
Cooper CR, Chay CH, Pienta KJ (2002) The role of αvβ3 in prostate cancer progression. Neoplasia 4:191–194
Pilch J, Habermann R, Felding-Habermann B (2002) Unique ability of integrin αvβ3 to support tumor cell arrest under dynamic flow conditions. J Biol Chem 277:21930–21938
Si Z, Hersey P (1994) Immunohistological examination of the relationship between metastatic potential and expression of adhesion molecules and ‘selectins’ on melanoma cells. Pathology 26:6–15
Brooks PC, Montgomery AM, Rosenfeld M, Reisfeld RA, Hu T, Klier G, Cheresh DA (1994) Integrin αvβ3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell 79:1157–1164
Sipkins DA, Cheresh DA, Kazemi MR, Nevin LM, Bednarski MD, Li KC (1998) Detection of tumor angiogenesis in vivo by αvβ3-targeted magnetic resonance imaging. Nat Med 4:623–626
Brooks PC, Stromblad S, Klemke R, Visscher D, Sarkar FH, Cheresh DA (1995) Anti-integrin αvβ3 blocks human breast cancer growth and angiogenesis in human skin. J Clin Invest 96:1815–1822
Gutheil JC, Campbell TN, Pierce PR, Watkins JD, Huse WD, Bodkin DJ, Cheresh DA (2000) Targeted antiangiogenic therapy for cancer using vitaxin: a humanized monoclonal antibody to the integrin αvβ3. Clin Cancer Res 6:3056–3061
Wu HR, Beuerlein G, Nie Y, Smith H, Lee BA, Hensler M et al (1998) Stepwise in vitro affinity maturation of vitaxin, an αvβ3 specific humanized MAB. Proc Natl Acad Sci USA 95:6037–6042
Mulgrew K, Kineer K, Yao XT, Ward BK, Damschroder MM, Walsh B, Mao SY, Gao C, Kiener PA, Coats S, Kinch MS, Tice DA (2006) Direct targeting of αvβ3 integrin on tumor cells with a monoclonal antibody, Abegrin. Mol Cancer Ther 5:3122–3129
World Health Organization (1979) WHO handbook for reporting results of cancer treatment (offset publication 48). World Health Organization, Geneva, Switzerland
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L et al (2000) New guidelines to evaluate the response to treatment in solid tumors. J Natl Can Inst 92:205–216 (Special Article)
Kuenen BC, Levi M, Miejers JC et al (2002) Analysis of the coagulation cascade and endothelial cell activation during inhibition of the VEGF/VEGF-receptor pathway in cancer patients. Arterioscler Thromb Vasc Biol 22:1500–1505
Faivre S, Delbaldo C, Vera K et al (2006) Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol 24:25–35
Gray R, Giantonio BJ, O’Dwyer PJ et al (2003) The safety of adding angiogenesis inhibition into treatment for colorectal, breast, and lung cancer: The Eastern Cooperative Oncology Group’s (ECOG) experience with bevacizumab (anti-VEGF). Proc Am Soc Clin Oncol 22:206 (Abstract 825)
Benson AB, Catalano PJ, Meropol NJ et al (2003) Bevacizumab (anti-VEGF) plus FOLFOX4 in previously treated advanced colorectal cancer (advCRC): An interim toxicity analysis of the Eastern Cooperative Oncology Group (ECOG) study E3200. Proc Am Soc Clin Oncol 22:243 (Abstract 975)
Patel SR, Jenkins J, Papadopoulous N et al (2001) Pilot study of vitaxin—an angiogenesis inhibitor—in patients with advanced leiomyosarcomas. Cancer 92:1347–1348
McNeel DG, Eickhoff J, Lee FT et al (2005) Phase I trial of a monoclonal antibody specific for alphavbeta3 integrin (MEDI-522) in patients with advanced malignancies, including an assessment of effect on tumor perfusion. Clin Cancer Res 11:7851–7860
Hersey P, Sosman S, O’Day J, Richards A, Bedikian R, Gonzalez W et al (2005) A phase II, randomized, open-label study evaluating the antitumor activity of MEDI-522, a humanized monoclonal antibody directed against the human alphavbeta3 integrin+dacarbazine (DTIC) in patients with metastatic melanoma (MM). Proc Am Soc Clin Oncol 24 (Abstract 7507)
Acknowledgments
Dr. Delbaldo was supported by a grant from the “Ligue Genevoise contre le Cancer”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Delbaldo, C., Raymond, E., Vera, K. et al. Phase I and pharmacokinetic study of etaracizumab (Abegrin™), a humanized monoclonal antibody against αvβ3 integrin receptor, in patients with advanced solid tumors. Invest New Drugs 26, 35–43 (2008). https://doi.org/10.1007/s10637-007-9077-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-007-9077-0