Abstract
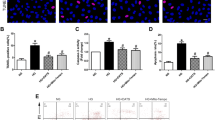
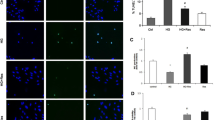
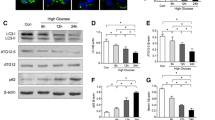
Excess apoptosis of endothelial cells (EC) plays crucial roles in the onset and progression of vasculopathy in diabetes mellitus. Anion exchanger-2 (AE2) might be involved in the vasculopathy. However, little is known about the molecular mechanisms that AE2 mediated the apoptosis of EC. The purpose of this study was to explore the role of AE2 in the apoptosis of HUVECs induced by high glucose (HG) and its possible mechanisms. First, HUVECs were exposed to different glucose concentrations (5.5, 17.8, 35.6, 71.2 and 142.4 mmol/l, respectively, pH = 7.40) for different time points (12, 24, 48, 72, 120, and 168 h, respectively). Intracellular Cl− concentration ([Cl−]i), AE2 expression and the apoptosis were assayed. Then, 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid (DIDS), Cl−-free media or specific RNA interference (RNAi) for AE2 was used to confirm whether AE2 could mediate the apoptosis induced by HG. Finally, the mechanisms of the AE2-mediated apoptosis were investigated by detecting mitochondrial permeability transition pore (mPTP, ΔΨm) openings, reactive oxygen species (ROS) levels and Caspase-3 activity. We found that HG upregulated the AE2 expression and activity, increased [Cl−]i and induced the apoptosis in a time- and concentration-dependent manner. The apoptosis of HUVECs by HG was possibly mediated by AE2 through an mPTP-ROS-Caspase-3 dependent pathway. These findings suggested that AE2 was likely to be a glucose-sensitive transmembrane transporter and a novel potential therapeutic target for diabetic vasculopathy.









Similar content being viewed by others
References
Ruderman NB, Williamson JR, Brownlee M (1992) Glucose and diabetic vascular disease. FASEB J 6:2905–2914
Pezet M, Verdetti J, Faury G (2004) Effect of glucose concentration on vascular function in aging: Action on calcium fluxes and vasomotricity induced by elastin peptides. J Soc Biol 198:279–286
The Diabetes Control and Complications Trial Research Group (1993) The effect of intensive treatment of diabetes on the development progression of long-term complications in insulin- dependent diabetes mellitus. N Engl J Med 329:977–986
UK Prospective Diabetes Study Group (1998) Intensive blood–glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352:837–853
Nakagami H, Kaneda Y, Ogihara T, Morishita R (2005) Endothelial dysfunction in hyperglycemia as a trigger of atherosclerosis. Curr Diabetes Rev 1:59–63
Isermann B, Vinnikov IA, Madhusudhan T et al (2007) Activated protein C protects against diabetic nephropathy by inhibiting endothelial and podocyte apoptosis. Nat Med 13:1349–1358
Nagaraj RH, Oya-Ito T, Bhat M, Liu B (2005) Dicarbonyl stress and apoptosis of vascular cells: prevention by alpha B-crystallin. Ann N Y Acad Sci 1043:158–165
Detaille D, Guigas B, Chauvin C et al (2005) Metformin prevents high-glucose-induced endothelial cell death through a mitochondrial permeability transition-dependent process. Diabetes 54:2179–2187
Sheu ML, Ho FM, Yang RS et al (2005) High glucose induces human endothelial cell apoptosis through a phosphoinositide 3-kinase-regulated cyclooxygenase-2 pathway. Arterioscler Thromb Vasc Biol 25:539–545
Varma S, Lal BK, Zheng R et al (2005) Hyperglycemia alters PI3 K and Akt signaling and leads to endothelial cell proliferative dysfunction. Am J Physiol Heart Circ Physiol 289:H1744–H1751
Ido Y, Carling D, Ruderman N (2002) Hyperglycemia-induced apoptosis in human umbilical vein endothelial cells: inhibition by the AMP-activated protein kinase activation. Diabetes 51:159–167
Cheng G, Shao Z, Chaudhari B, Agrawal DK (2007) Involvement of chloride channels in TGF-B1-induced apoptosis of human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 293:L1339–L1347
Lang F, Föller M, Lang K et al (2007) Cell volume regulatory ion channels in cell proliferation and cell death. Methods Enzymol 428:209–225
Guan YY, Wang GL, Zhou JG (2006) The ClC-3 Cl-channel in cell volume regulation, proliferation and apoptosis in vascular smooth muscle cells. Trends Pharmacol Sci 27:290–296
Shiio Y, Suh KS, Lee H, Yuspa SH, Eisenman RN, Aebersold R (2006) Quantitative proteomic analysis of myc-induced apoptosis: a direct role for Myc induction of the mitochondrial chloride ion channel, mtCLIC/CLIC4. J Biol Chem 281:2750–2756
Lemonnier L, Shuba Y, Crepin A et al (2004) Bcl-2-dependent modulation of swelling-activated Cl- current and ClC-3 expression in human prostate cancer epithelial cells. Cancer Res 64:4841–4848
Varela D, Simon F, Riveros A, Jørgensen F, Stutzin A (2004) NAD(P)H oxidase-derived H2O2 signals chloride channel activation in cell volume regulation and cell proliferation. J Biol Chem 279:13301–13304
Shennan DB (2008) Swelling-induced taurine transport: relationship with chloride channels, anion-exchangers and other swelling-activated transport pathways. Cell Physiol Biochem 21:15–28
Faber S, Lang HJ, Scholkens BA, Mutschler E (1998) Intracellular pH regulation in bovine aortic endothelial cells: evidence of both Na+/H+ exchange and Na+-dependent Cl-/HCO3-exchange. Cell Physiol Biochem 8:202–211
Alper SL (2006) Molecular physiology of SLC4 anion exchangers. Exp Physiol 91:153–161
Liu CJ, Hwang JM, Wu TT et al (2008) Anion exchanger inhibitor DIDS induces human poorly-differentiated malignant hepatocellular carcinoma HA22T cell apoptosis. Mol Cell Biochem 308:117–125
Fujita H, Ishizaki Y, Yanagisawa A, Morita I, Murota SI, Ishikawa K (1999) Possible involvement of a chloride-bicarbonate exchanger in apoptosis of endothelial cells and cardiomyocytes. Cell Biol Int 23:241–249
Huang Q, He M, Chen H et al (2007) Protective effects of sasanqua-saponin on injury of endothelial cells induced by anoxia and reoxygenation in vitro. Basic Clin Pharmacol Toxicol 101:301–308
Reynolds A, Leake D, Boese Q et al (2004) Rational siRNA design for RNA interference. Nat Biotechnol 22:326–330
Ui-Tei K, Naito Y, Takahashi F et al (2004) Guidelines for the selection of highly effective siRNA sequences for mammalian and chick RNA interference. Nucleic Acids Res 32:936–948
Zang M, Gong J, Luo L et al (2008) Characterization of S338 phosphorylation for Raf-1 activation. J Biol Chem 283:31429–31437
Lai ZF, Shao Z, Chen YZ, He M, Huang Q, Nishi K (2004) Effects of sasanquasaponin on ischemia and reperfusion injury in mouse hearts. J Pharmacol Sci 94:313–324
Alvarez BV, Fujinaga J, Casey JR (2001) Molecular basis for angiotensin II-induced increase of chloride/bicarbonate exchange in the myocardium. Circ Res 89:1246–1253
Mardones P, Medina JF, Elferink RP (2008) Activation of cyclic AMP signaling in Ae2-deficient mouse fibroblasts. J Biol Chem 283:12146–12153
Ghio AJ, Grayck EN, Turi J et al (2003) Superoxide-dependent iron uptake: a new role for anion exchanger protein 2. Am J Respir Cell Mol Biol 29:653–660
Stewart AK, Kurschat CE, Vaughan-Jones RD, Alper SL (2009) Putative re-entrant loop 1 of AE2 transmembrane domain has a major role in acute regulation of anion exchange by pH. J Biol Chem 284:6126–6139
Humphreys BD, Jiang L, Chernova MN, Alper SL (1995) Hypertonic activation of AE2 anion exchanger in Xenopus oocytes via NHE-mediated intracellular alkalinization. Am J Physiol 268:C201–C209
Frische S, Zolotarev AS, Kim YH et al (2004) AE2 isoforms in rat kidney: immunohistochemical localization and regulation in response to chronic NH4Cl loading. Am J Physiol Renal Physiol 286:F1163–F1170
Nickell WT, Kleene NK, Kleene SJ (2007) Mechanisms of neuronal chloride accumulation in intact mouse olfactory epithelium. J Physiol 583:1005–1020
Alper SL (2009) Molecular physiology and genetics of Na+-independent SLC4 anion exchangers. J Exp Biol 212:1672–1678
Chernova MN, Stewart AK, Jiang L, Friedman DJ, Kunes YZ, Alper SL (2003) Structure–function relationships of AE2 regulation by Ca(i)(2+)-sensitive stimulators NH(4+) and hypertonicity. Am J Physiol Cell Physiol 284:C1235–C1246
Stewart AK, Kurschat CE, Burns D, Banger N, Vaughan-Jones RD, Alper SL (2007) Transmembrane domain histidines contribute to regulation of AE2-mediated anion exchange by pH. Am J Physiol Cell Physiol 292:C909–C918
Li Y, Wu H, Khardori R, Song YH, Lu YW, Geng YJ (2009) Insulin-like growth factor-1 receptor activation prevents high glucose-induced mitochondrial dysfunction, cytochrome-c release and apoptosis. Biochem Biophys Res Commun 384:259–264
Chen G, Shen X, Yao J et al (2009) Ablation of NF-kappaB expression by small interference RNA prevents the dysfunction of human umbilical vein endothelial cells induced by high glucose. Endocrine 35:63–74
Dong Z, Wang J, Zhong Q (2003) Postmitochondrial regulation of apoptosis by bicarbonate. Exp Cell Res 288:301–312
Fujita H, Morita I, Murota S (2000) Hydrogen peroxide induced apoptosis of endothelial cells concomitantly with cycloheximide. J Atheroscler Thromb 7:209–215
Acknowledgments
This work was supported by grants from the Natural Scientific Foundation of China (No. 30660058 and No. 30860111). We thank Dr. Huixin Deng, Xuan Jin, Shiwen Luo and Gregory D. Jensen for generous help in correcting the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Qing Li, Yuan-Hong Chen and Li Li are equally contributed to this article.
Rights and permissions
About this article
Cite this article
Huang, QR., Li, Q., Chen, YH. et al. Involvement of anion exchanger-2 in apoptosis of endothelial cells induced by high glucose through an mPTP-ROS-Caspase-3 dependent pathway. Apoptosis 15, 693–704 (2010). https://doi.org/10.1007/s10495-010-0477-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-010-0477-9