Abstract
A great deal of literature has drawn attention to the “complex Chiari,” wherein the presence of instability or ventral brainstem compression prompts consideration for addressing both concerns at the time of surgery. This report addresses the clinical and radiological features and surgical outcomes in a consecutive series of subjects with hereditary connective tissue disorders (HCTD) and Chiari malformation. In 2011 and 2012, 22 consecutive patients with cervical medullary syndrome and geneticist-confirmed hereditary connective tissue disorder (HCTD), with Chiari malformation (type 1 or 0) and kyphotic clivo-axial angle (CXA) enrolled in the IRB-approved study (IRB# 10-036-06: GBMC). Two subjects were excluded on the basis of previous cranio-spinal fusion or unrelated medical issues. Symptoms, patient satisfaction, and work status were assessed by a third-party questionnaire, pain by visual analog scale (0–10/10), neurologic exams by neurosurgeon, function by Karnofsky performance scale (KPS). Pre- and post-operative radiological measurements of clivo-axial angle (CXA), the Grabb-Mapstone-Oakes measurement, and Harris measurements were made independently by neuroradiologist, with pre- and post-operative imaging (MRI and CT), 10/20 with weight-bearing, flexion, and extension MRI. All subjects underwent open reduction, stabilization occiput to C2, and fusion with rib autograft. There was 100% follow-up (20/20) at 2 and 5 years. Patients were satisfied with the surgery and would do it again given the same circumstances (100%). Statistically significant improvement was seen with headache (8.2/10 pre-op to 4.5/10 post-op, p < 0.001, vertigo (92%), imbalance (82%), dysarthria (80%), dizziness (70%), memory problems (69%), walking problems (69%), function (KPS) (p < 0.001). Neurological deficits improved in all subjects. The CXA average improved from 127° to 148° (p < 0.001). The Grabb-Oakes and Harris measurements returned to normal. Fusion occurred in 100%. There were no significant differences between the 2- and 5-year period. Two patients returned to surgery for a superficial wound infections, and two required transfusion. All patients who had rib harvests had pain related that procedure (3/10), which abated by 5 years. The results support the literature, that open reduction of the kyphotic CXA to lessen ventral brainstem deformity, and fusion/stabilization to restore stability in patients with HCTD is feasible, associated with a low surgical morbidity, and results in enduring improvement in pain and function. Rib harvest resulted in pain for several years in almost all subjects.
Similar content being viewed by others
Introduction
Many studies have drawn attention to the presence of craniocervical instability or basilar invagination in patients with Chiari one and Chiari zero malformation [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23]. The need for reduction and stabilization in basilar invagination and craniocervical instability are recognized in connective tissue joint degenerative disorders, such as rheumatoid arthritis and lupus [10, 17, 24,25,26,27,28,29,30,31,32,33,34,35,36] and hereditary hypermobile and developmental disorders, including osteogenesis imperfecta, achondroplasia, Down syndrome and Ehlers-Danlos syndrome (EDS) [8, 18, 21, 26, 31, 37,38,39,40,41,42,43,44,45,46,47,48,49,50].
Emblematic of the approximately 50 heritable connective tissue disorders characterized by joint hypermobility is Ehlers-Danlos syndrome (EDS). Though Ehlers-Danlos syndrome was described in 1905, its neurological and spinal manifestations have only recently been appreciated [18, 41, 51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66]. These heritable connective tissue disorders are characterized by tissue fragility, skin extensibility, joint hypermobility, premature disk degeneration and spinal problems, and numerous comorbid conditions.
We report on an IRB-approved retrospective cohort study of 20 consecutive patients with hereditary connective tissue disorders and a kyphotic CXA, cerebellar ectopia (18/20), and craniocervical instability or ventral brainstem compression, who underwent reduction and stabilization. This is the first such study to critically assess 5-year outcomes after craniocervical reduction, stabilization, and fusion in a patient population with hereditary connective tissue disorders.
In this study, the CXA (clivo-axial angle) was used to indicate potential brainstem deformity. The CXA has drawn increasing attention as an important radiological metric to indicate the presence of neurological deficit and consideration for craniocervical stabilization [4]. The line of reasoning that a kyphotic CXA is associated with pathologic bending of the brainstem (medullary kyphosis, or kink) began with Liszt, who first recognized that clivo-axial kyphosis may result in neurobehavioral effects. Van Gilder reported that CXA of less than 150° were often associated with neurological deficits [67]. Breig demonstrated the importance of mechanical tension and deformation of the brainstem [68]. Menezes described the “fulcrum effect in basilar invagination, by which traction is applied to the caudal brainstem and rostral cervical spinal cord. Others have demonstrated the salutary consequences to the correction of the CXA [1, 10, 12, 15, 30, 49, 69,70,71,72,73,74,75].
It is important to recognize that the CXA is simply a static representation of a dynamic phenomenon. It has been generally considered that a CXA of less than 135° represents the threshold below which chronic repetitive injury may occur as a result of mechanical deformation of the lower brainstem and upper spinal cord.
The authors’ hypothesis was that reduction of the Clivo-axial kyphosis and stabilization for craniocervical instability were feasible and associated with clinical improvement in the hereditary connective tissue disorder (HCTD) population.
Materials and methods
Subject enrollment
Over a 2-year period (2011–2012), a cohort of 22 consecutive patients diagnosed with EDS, or in a few cases, unspecified hereditary connective tissue disorders (HCTD), were enrolled in the study and underwent occipital to C1/C2 fusion for craniovertebral instability and flexion deformity. Of the original 22 consecutive subjects, two were excluded: one had previously undergone a cranio-spinal fusion, and the second declined to participate due to unrelated medical issues. The data analysis was, therefore, conducted on the remaining 20 subjects, all of whom were enrolled in the IRB-approved study (IRB# 10-036-06: Greater Baltimore Medical Center). In 18 patients, cerebellar ectopia was also present.
Evaluation
Symptoms were assessed by a standardized questionnaire administered by third party at 2 and 5 years. Pain was assessed by the visual analog scale for pain (0–10/10). The neurologic exams were performed by the neurosurgeon. Function and the ability to return to work were assessed with the Karnofsky Performance Scale (Fig. 1). Radiological measurements were performed by a neuroradiologist (MK) after 2 years.
Pre- and post-operative radiological measurements were made or reviewed by the neuroradiologist (MK). Subjects underwent pre-operative and post-operative imaging with MRI and CT of the cervical spine. Upright, weight-bearing flexion and extension MRI of the cervical spine was obtained in 10/20 of the subjects.
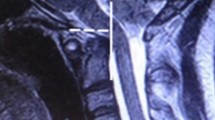
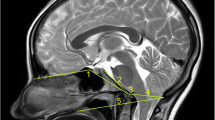
Radiometrics were performed at the 2-year follow-up and included the clivo-axial angle (CXA), Grabb-Mapstone-Oaks measurement (the pBC2), and the horizontal Harris Measurement (Basion axis interval or BAI). CXA is the measurement in degrees between the line drawn along the lower third of the clivus, and a line drawn along the posterior aspect of the axis [1, 76] (Fig. 2a). The CXA measurements were taken from the flexion image, when it was available (Fig. 2b, c).
a The normal CXA. The normal CXA is approximately 155°, decreasing 10° in flexion and increasing 10° in extension. b The pathological clival axial angle (CXA) is more kyphotic than the normal CXA. The CXA is subtended by the posterior axial line and a line drawn along the surface of the lower third of the clivus. An angle of 135° or less is considered potentially pathological. The kyphotic CXA of 124° shown here is clearly pathological and results in a mechanical deformity and lengthening of the brainstem and upper spinal cord, as shown diagrammatically in the next image (Fig. 2c). c Diagrammatical rendering of a kyphotic CXA. In hereditary connective tissue disorders, ligamentous laxity may thus result in a kyphotic CXA in flexion, with a concurrent increase in strain (Ɛ)
The pBC2, or Grabb, Oakes measurement (Fig. 3) is the perpendicular measurement from the dura to a line drawn from the basion to the posterior inferior aspect of C2 [7, 76, 77].
Horizontal Harris measurement or BAI is the distance from the basion drawn perpendicularly to the posterior axial line (PAL) (Fig. 4). A measurement greater than 12 mm represents instability [76,77,78]. When possible, the Harris measurement/BAI is made from the MRI or CT in both flexion and extension to assess translation (sliding movement) between flexion and extension.
Inclusion criteria for occipital-cervical fusion stabilization surgery
All subjects met the following criteria:
- i.
Formal genetics evaluation and diagnosis with a hereditary connective tissue disorder (CF)
- ii.
Signed consent
- iii.
Severe headache and/or neck pain greater than or equal to 7/10 by the visual analog scale for greater than 6 months.
- iv.
- v.
Demonstrable neurological deficits
- vi.
Congruent radiological findings were in accordance with the treatment algorithm previously set forward [70], including kyphotic CXA (less than 135°), craniocervical instability (greater than Harris/BAI measurement of 4 mm*), or low-lying cerebellar tonsils or Chiari malformation.
- vii.
Failed conservative treatment (physical therapy, activity modification, pain medications, neck brace, and in some circumstances, chiropractic, electrical stimulation, massage)
*Note: The normal Harris/BAI measurement changes no more than 1 mm between flexion and extension. The authors allowed 3 mm for error.
Operative technique
Preoperative traction reduction was not performed. Subjects were intubated in the neck brace with a GlideScope intubation technique to improve the view of the glottis and to avoid hyperextension of the neck. Sensory evoked potentials were performed throughout the surgery. A three-pronged Mayfield head holder was placed, and the subject positioned prone on chest rolls. The cervical spine was carefully aligned to eliminate tilt and rotation, and then placed in a neutral position, as confirmed by cross table fluoroscopy. After sterile prep and drape, the incision was made from inion to C4, but the subperiosteal exposure was limited to the occiput, C1and C2. Care was taken to preserve the ligaments attached to the dorsal aspect of the spinous process of C2 and to the caudal aspect of the C2 lamina.
A limited sub occipital decompression was performed with high speed burr and Kerrison rongeur from the foramen magnum upward 14 mm, but carried laterally to the full meridian of the dura. The dura was not opened, and thus, no expansion duroplasty was performed.
Open reduction of the craniocervical junction was performed to normalize the CXA. To accomplish the open reduction, the surgeon stepped to the head of the table, applied traction, posterior translation, and extension at the craniocervical junction. The head holder was then locked in place and checked with fluoroscopy (Fig. 5). Sensory and motor-evoked potentials were continuously monitored throughout the procedure. The reduction was accomplished in one to four iterations, under fluoroscopic guidance, with the goal of increasing the CXA by approximately 20° [10, 12, 70] and to bring the basion over the midpoint or anterior half of the odontoid (Fig. 6).
These subjects underwent a craniocervical fusion and stabilization in order to maintain the corrected CXA and relationship of the basion to the odontoid process and to stabilize the craniocervical junction. To accomplish the stabilization, a titanium plate (Nex-Link OCT® Occipital cervical plating system, Zimmer) was contoured slightly and affixed to the occiput. Titanium 3.5-mm screws were placed in the C1 lateral masses and the C2 pedicles bilaterally. After reduction, the screws were connected by rods to the occipital plate [80,81,82]. In one case, it was necessary to place screws in the C3 lateral masses to achieve adequate stability.
To accomplish the fusion, bone surfaces were decorticated. Two rib autografts were harvested at approximately the T7 level [83]. The rib grafts were contoured to fit from the suboccipital bone to the upper cervical vertebrae, augmented with demineralized bone matrix, and secured with number one proline to prevent migration of the graft.
Both the neck and graft harvest wounds were then closed over drains. The patients were usually mobilized 1 day after surgery and kept in a neck brace (Miami J™, or equivalent) for 4 weeks. Physical therapy was then started.
Statement of human and animal rights
All procedures performed in studies involving human participants were carried out in accordance with the ethical standards of the institutional and/or national research committee in the United States, and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual patients and participants included in the study.
Results
Nineteen subjects were female and one male, with an average age of 24 years (range of 12–53 years). All patients were diagnosed with a hereditary connective tissue disorder (HCTD): ten had hypermobile EDS (h-EDS), two classical EDS, four unspecified EDS, and four hypermobility spectrum disorder. All subjects (20/20) had a kyphotic CXA (less than or equal to 135°) and craniocervical instability (Harris Measurement/BAI of 4 mm or greater). Eighteen subjects had cerebellar ectopia.
Pre-operative findings
The most prominent symptoms prior to surgery included headache (100%), fatigue (100%), dizziness (100%), muscle pain, vertigo, arm weakness, neck pain, balance problems, memory problems, night awakenings, numbness and weakness of the arms and legs, and gait problems (Table 1).
Patient satisfaction
There was 100% follow-up at 2 years and 5 years (Figs. 7 and 8). All patients were satisfied with the surgery and would repeat the surgery given similar circumstances, and reported improved quality of life (Figs. 9, 10, and 11). All but one patient would recommend the surgery to a family member (Fig. 10). Eighteen of the twenty patients reported that the craniocervical fusion surgery had decreased their limitations; the remaining two patients, who responded that the limitations had not decreased with surgery, explained that there remained limitations from other medical problems and spinal instability elsewhere (Fig. 12).
Postoperative findings
Postoperatively at 2 years, statistically significant improvements were seen in vertigo (92%), headaches (85%), imbalance (82%), dysarthria (80%), dizziness (70%), memory (69%), walking (69%), and frequent daytime urination (42%) (Table 1). The average headache decreased from 8.1/10 pre-op to 4.35/10 post-op (p < 0.0001). Neck pain mean decreased in 71% of patients, from 6.45/10 to 4.05/10 post-op (p < 0.002), and muscle pain decreased from 6/10 to 4.7/10 post-op (p < 0.009) (Table 2).
Improvement, though not statistically significant, included tremors (87%), syncope (86%), numbness of the arms and hands (73%), upper extremity numbness (73%), lower extremity weakness (69%), back numbness (67%), swallowing difficulty (63%), upper extremity weakness (61%), hearing problems (61%), lower extremity numbness (55%), and GERDS (55%) (Table 1).
Similarly, at 5 years, there remained statistically significant improvement in dizziness (75%), walking problems (69%), speech problems (67%), frequent daytime urination (67%), headaches (65%), and imbalance (59%). Improvement in upper extremity numbness, syncope, lower extremity weakness, back numbness, swallowing difficulty, upper extremity weakness, hearing problems, and lower extremity numbness were improved but not with statistical significance (Tables 2, 3, 4, and 5).
On neurological examination, those who were weak before surgery improved, though not completely. The ability to walk heel-to-toe, Romberg, and sensation were all improved. There was no significant improvement in reflexes (Table 3).
Functional outcome
Function and the ability to return to work, as assessed with the Karnofsky Performance Scale, demonstrated a highly statically significant improvement (p < 0.001). Preoperatively, 12/20 subjects were completely disabled, and 4/20 were able to care for themselves only, but unable to go to work or school. Postoperatively, 3/20 showed no change and 3/20 worsened on the Karnofsky scale. However, 14/20 subjects improved in their Karnofsky score: 5/20 had improved in work/school status, and an additional two subjects were seeking part time work or about to begin school, for a total of 7/20. Many patients were able to return to caring for their families and enjoying life to some extent; overall, 10/20 had a Karnofsky of 80 or higher (Fig. 7, Table 6).
Karnofsky scores were reassessed post-operatively at 5 years. There remained statistically significant improvement (p < 0.003). Eleven of 20 patients remained in work or school; 17/20 had improvement in Karnofsky compared to pre-op, 1/20 had no change and 2/20 had worsened (Fig. 7).
There was no significant difference found between the 2-year and 5-year Karnofsky (p < 0.43) (Fig. 7). Compared to the 2-year score, the 5-year post-op Karnofsky evaluation had improved in 8/20, showed no change in 6/20, and worsened in 6/20.
Radiological outcomes
Open reduction was successful in normalizing the CXA in every subject. Preoperatively, radiological examination demonstrated abnormal CXA (less than or equal to 135°) in 20/20 subjects, with an average CXA of 127° (Fig. 8). Post-operatively at 2 years, the average CXA was 148° (p < 0.001).
Preoperatively, the Grabb, Mapstone, Oakes measurement was made in 18 subjects; the methodology yielded a measurement greater than 9 mm in 9/18 subjects, constituting a high-risk category for ventral brainstem compression [7]. Postoperatively at 2 years, all subjects (20/20) were within the normal range (less than 9 mm).
Preoperatively, the horizontal Harris measurement demonstrated craniocervical instability in 5/6 patients; in these patients, there was pathological translation varying from a mean of 4 to 9 mm. Translation in the Harris measurement was the difference between that measured on flexion and that measured on extension in the upright MRI [76,77,78]. Post-operatively, as a consequence of the reduction and stabilization, the translation by horizontal Harris measurement was less than or equal to 1 mm in 12 out of 14 subjects and equal to 2 mm in 2 out of 14 subjects (Table 4).
Eleven of twenty had Chiari malformation (descent of the cerebellar tonsils of 5 mm or more below McRae’s Line), of whom, five had undergone a prior suboccipital decompression; one had a Chiari Zero; six subjects had low-lying cerebellar tonsils (cerebellar ectopia, where the descent of the cerebellar tonsils did not reach the 5 mm threshold).
The fusion rate as determined by postoperative CT scan was 100%.
Complications of surgery
There were no deaths or major peri-operative morbidities. Two subjects underwent transfusion intraoperatively. Two subjects had superficial infections, of which one returned to the operating room for closure of the rib wound dehiscence. Mild to moderate pain (3/10) at the rib harvest site was common at 2 years, substantially abating at 5 years.
Despite the loss of 20 to 30° of flexion and extension at the craniocervical junction, and 35° of rotation to each side at C1–C2, range of motion was not a concern for any of these subjects. One to four years after the craniocervical fusion, some subjects developed pain over the suboccipital instrumentation (the “screw saddles”) due to tissue thinning, and requested hardware removal (8/20 subjects).
Discussion
This is the first 5-year study to retrospectively examine the outcome of craniocervical fusion in patients with a hereditary connective tissue disorder and craniovertebral instability. The study reviews responses of a cohort of 20 subjects disabled with pain and neurologic deficit, who had failed non-operative regimens, who presented with kyphotic clivo-axial angle (CXA less than 135°) and basilar invagination, or instability at the craniocervical junction (CCI) in the setting of a hereditary connective tissue disorder, such as Ehlers-Danlos syndrome. Eighteen of the twenty subjects had low-lying cerebellar tonsils, including Chiari malformation, type I or type 0.
Ehlers-Danlos syndrome
Emblematic of the approximately 50 hereditary connective tissue disorders are the Ehlers-Danlos syndromes (EDS), a heterogeneous group of heritable, connective tissue disorders characterized by joint hypermobility, skin extensibility, and tissue fragility. The 2017 classification [84] recognizes 13 subtypes, which for the most part are due to mutation of genes that encode fibrillary collagens or the enzymes involved in post-translational modification of collagen. Hypermobile type EDS (h-EDS) is diagnosed on the basis of clinical findings [85], while molecular testing is available to confirm most other forms of EDS [84, 86,87,88]. The neurological and spinal manifestations of h-EDS and the classic form of EDS have been reviewed [41, 89, 90].
Ligamentous laxity at the craniocervical junction
EDS is fundamentally a disorder of collagen and other structural components of connective tissue, characterized by incompetent ligaments, joints, and spine. Ligaments are the major occiput–C1 stabilizing structures [4]. In the presence of ligamentous laxity or disruption, the CCJ is incompetent in the execution of multiaxial movements [91, 92]. Craniocervical instability (CCI) is thus a manifestation of ligamentous laxity in EDS [18, 53, 61, 62, 93, 94].
Most atlanto-occipital joint movement occurs in flexion-extension, and axial rotation is normally limited; greater than 5° rotation at the occipito-atlantal joint is abnormal [95]. The lateral atlanto-occipital ligament prevents excess rotation between occiput and atlas; incompetence of the lateral atlanto-occipito ligament results in increased contralateral rotation by 3 to 5°. The tectorial membrane and nuchal ligament, composed parallel bundles of collagen, restrict hyperflexion, maintain posture, and help to restore normal position [96]. In the population of patients with hypermobility connective tissue disorders, incompetent ligamentous connections from the skull to the spine may progress to CCI.
Neurological deficit has been attributed to ligamentous laxity at the craniocervical junction
Neurological injury is common in many other connective tissue disorders, such as rheumatoid arthritis, Down syndrome, and hereditary disorders such asosteogenesis imperfecta [10, 17, 18, 21, 25,26,27,28, 30,31,32,33,34,35, 37, 42, 44,45,46, 48, 49]. Non-disruptive stretch injury of the neuroaxis has been attributed to hypermobility of the craniocervical junction in infants and children, in whom the axonal lesions tend to be localized to the dorsal brainstem, lower medulla, in particular the corticospinal tracts at craniocervical junction [97]. Similar histopathological findings of nerve injury were seen in the lower brainstem and spinal cord, in adults [30, 71, 98,99,100].
In the EDS population, motor delay, developmental coordination disorder, headaches secondary to spinal compression, clumsiness, and the relatively high rate of dyslexia and dyspraxia have been recognized as a consequence of the effects of ligamentous laxity upon the central nervous system [18, 51, 53,54,55,56,57,58,59,60, 62, 66]. Interdigitation of the posterior-atlanto-occipital membrane with the pain-sensitive spinal dural layer has also been implicated in the genesis of headache [101].
The cervical medullary syndrome
The cervical medullary syndrome, also known as “craniocervical syndrome” (ICD-9-CM Diagnosis Code 723.2; ICD-10-CM Diagnosis Code M53.0), comprises those symptoms commonly attributed to lower brainstem and upper cervical spinal cord pathology, usually in the presence of a “complex Chiari” (Chiari malformation with basilar invagination or craniocervical instability) [1, 3,4,5, 77, 79].
In the present study, all subjects presented with headache, fatigue and dizziness, and most reported, in descending order of frequency: weakness, neck pain, imbalance, night awakenings, memory difficulties, walking problems, sensory changes, visual problems, vertigo, altered hearing, speech impediments, micturition issues and dysphagia, and syncopal episodes. In aggregate, these symptoms are reasonably described as the “Cervical Medullary Syndrome” [1, 77].
While there is an overlap of clinical findings, the clinical presentation of the pure Chiari malformation differs from the complex Chiari malformation. Chiari I malformations are characterized primarily by the suboccipital “cough headache” exacerbated by Valsalva, cough or straining-dizziness, elements of cerebellar dysfunction, lower cranial nerve deficits, and gait problems [102]. On the other hand, the “Complex Chiari” with ventral brainstem compression or craniocervical instability present with other genetic conditions—such as HOX D3 homeotic transformation, Klippel Feil malformation, hereditary connective tissue disorders [102,103,104]—and is characterized by pyramidal changes, with weakness, hyperreflexia, pathological reflexes, paresthesias, and sphincter problems, in addition to headache, neck pain, dizziness, vertigo, dyspnea, dysphonia, altered vision and hearing, syncope, gait changes, and altered sleep architecture [5, 7, 10, 30, 70, 71, 105,106,107]. Dysautonomia has also been attributed to basilar impression [108].
Radiological metrics in the diagnosis of basilar invagination and CCI
Three radiologic metrics used in this study, the Clivo-axial angle (CXA), the horizontal Harris Measurement [78], and the Grabb, Mapstone, Oakes measurement [7, 78] have been adopted as common data elements (CDEs) by the NIH/NINDS, and characterized useful in identifying possible CCI and basilar invagination [1, 76, 77]. The CXA of less than 135° is considered potentially pathological [10, 12, 18, 30, 70,71,72,73,74,75, 79, 109]. Salutary consequences have been attributed to the correction of the CXA [10, 12, 69, 70, 107].
The Grabb, Mapstone, Oakes measurement of 9 mmor more suggests high risk of ventral brainstem compression, requiring consideration for craniospinal reduction or transoral decompression, and fusion stabilization [7, 77, 79].
The horizontal Harris measurement (or BAI) was useful in demonstrating craniocervical instability. Normally, the basion pivots on a point above the odontoid, and there is no measurable translatory movement between flexion and extension. A change in the horizontal Harris measurement of 2 mm or more, as measured in flexion and extension images, represents pathological translation between the basion and odontoid [1, 10, 76, 77, 79, 110,111,112,113,114].
Non-operative management of patients with craniocervical instability due to hereditary connective tissue disorder
Patients should be given a specific diagnosis to validate their concerns, and allay their fears. Rigorous instruction should follow to avoid aggravating activities—impact sports and prolonged sitting or driving, the importance of frequent rest periods, physical therapy—for strengthening, sagittal balance, posture and cardiorespiratory fitness, and judicious use of appropriate bracing, to be accompanied by isometric exercises. When possible, treatment of co-morbid conditions should be undertaken.
Craniocervical fusion should be considered the last option, to be engaged when non-operative treatment has failed.
Indications for surgery
Posterior occipito-cervical fusion is indicated in patients who present with basilar invagination, instability or abnormal biomechanics, and cervical medullary syndrome [13, 21, 25, 112, 115].
Therefore, at the time of decompression of a Chiari malformation, the finding of basilar invagination or craniocervical instability should prompt consideration of fusion and stabilization [2, 3, 11, 18, 19, 21,22,23, 116, 117].
In this study, indications for surgery included disabling headache or neck pain, symptoms constituting the cervical medullary syndrome with demonstrable neurological findings, congruent radiological findings, a determination on the part of the patient that they were unable to continue in the normal activities of daily living, and failed non-operative treatment.
Headache should not be attributed a priori to craniocervical instability. In the hereditary connective tissue disorders, headache may have many origins: cervicogenic, vessel dissection, or venous occlusive disease or thrombosis, intracranial hypertension or hypotension, temporomandibular joint syndrome, inflammatory and infectious disorders, neuralgia and migrainous conditions, postural orthostatic tachycardia syndrome (POTS), or mast cell activation syndrome (MCAS) [41, 118, 119].
Radiological metrics are useful guidelines, but not indications, per se, for surgery. The radiological indications were congruent with the treatment algorithm previously established [70]. Abnormal radiological metrics may exist in patients with no neurological symptoms.
A number of subjects with CCI were also found to have atlantoaxial instability, a radiological and clinical finding that did add weight to the decision to proceed to surgery. Occipitocervical fusion is indicated in some circumstances for atlantoaxial instability alone, or for complex cervical deformities [21, 27].
A patient with hereditary disorder is at risk for multilevel instability issues; any injury or period of disability may result in exacerbation of instability [120]. The complexity of these patients warrants a rigorous selection process. Selection of candidates for surgery should follow standard guidelines and indications for instability, the diagnosis of which often requires dynamic imaging [13, 14, 70]. Occipitocervical fusion should be considered the last treatment option in this patient population [41].
Surgical open reduction
The reduction should be executed in a thoughtful and deliberate manner to avoid incorrect or painful malalignment, “star gazing” from excessive extension or conversely a downward gaze. If the cranium is inadequately extended, the oropharyngeal space may be decreased, and the patient may exhibit severe dysphagia or potentially life-threatening dyspnea [121]. To maintain appropriate oropharyngeal space, the surgeons extended the cervical spine to maintain 2 cm between the anterior spinal line and the posterior edge of the mandible, as seen on lateral fluoroscopy. In most cases, the basion was translated posteriorly to lie above the midpoint of the odontoid. The kyphotic angulation of the brainstem over the odontoid process, as measured by the CXA, was normalized by extension of the cranium at the craniocervical junction, thereby decreasing the fulcrum effect of the odontoid [49], and the mechanical stress on the brainstem [10, 12, 30, 41, 109, 122]. We attempted to achieve a mild cervical lordosis.
Reduction, fusion/stabilization appears to improve pain and neurological deficit
There was 100% follow-up at 2-year and 5-year follow-up. Except for the neurological exam, the clinical data was collected by a third party, and de-identified. All patients were satisfied with the surgery, would repeat the surgery given the same circumstances, and reported improved quality of life. All but one patient would recommend the surgery to a family member. Eighteen of the twenty patients reported that the craniocervical fusion surgery decreased their limitations; two reported continued limitations from other medical problems and spinal instability elsewhere.
Postoperatively, at the 2-year follow-up, patients demonstrated a statistically significant improvement in in frequency and severity of headache, speech, memory, vertigo, dizziness, gait, balance, and urinary frequency. There were also improvements in most patients with tremors, syncope, imbalance, hearing problems, dysarthria, swallowing difficulty, numbness of the upper and lower extremities and back, neck pain and upper extremity weakness.
At 5 years, there remained statistically significant improvement in headaches, dizziness and imbalance, gait, speech problems, and frequent daytime urination. Though not statistically significant, there was also continued improvement in upper extremity, back and lower numbness, syncope, upper and lower extremity weakness, swallowing difficulty, and hearing problems.
At the 2-year period, the improvement of the Karnofsky performance score was statistically significant and remained significantly improved over the 5-year follow-up period, with the majority of subjects returning to employment, school, or work in the home. This improvement was supported by the observed improvement in neurological deficits; weakness, heel-to-toe walking, Romberg and sensation.
Co-morbid conditions in this population that confounded the outcome
At 5 years, 8/20 patients reported disability from co-morbid conditions. In keeping with the literature, most patients presented with postural orthostatic tachycardia syndrome and other manifestations of dysautonomia; many patients received diagnoses of abnormalities of CSF hydrodynamics with intracranial hypertension or hypotension, abnormalities of intracranial venous drainage due to sinus stenosis or jugular vein stenosis. Migraine headaches and temporomandibular joint dysfunction were very common. A majority of patients had vitamin and trace element deficiencies. Many patients demonstrated cervical instability with cervicogenic headaches. Gastroparesis, superior mesenteric artery syndrome, mast cell activation syndrome occurred and endocrine disorders. Several patients were diagnosed with movement disorders, Tarlov cysts, kypho-scoliosis, tethered cord syndrome, neuromuscular disorders, anxiety, and depression [18, 41, 123,124,125,126,127,128,129].
A multi-disciplinary team, familiar with the many co-morbidities and the generalized ligament laxity throughout the spinal column, is necessary to address the many issues in order to improve the well-being of the patient with a hereditary connective tissue disorder.
Complications of surgery
There were no deaths or major peri-operative morbidities. There were two patients who underwent transfusion intraoperatively, two with superficial infections of whom one returned to the operating room for closure of the rib wound dehiscence. Mild to moderate pain at the rib harvest site was common at 2 years, substantially abating at 5 years. Spinal instability is a potential complication of rib harvest, but was not reported in this group.
The absence of screw malposition and vertebral artery injury [29, 130] is attributed in part to improvement in instrumentation, preoperative CT to examine the anatomy, and intra-operative fluoro-CT to assess the construct real-time.
No patient complained of decreased neck range of motion after surgery. Despite the loss of approximately 20° to 30° of flexion and extension at the craniocervical junction, and 35° of rotation to each side at C1–C2, range of motion was not a concern for any of these patients.
One to four years after the craniocervical fusion, some patients developed pain over the suboccipital instrumentation (the “screw saddles”) due to tissue thinning, and requested hardware removal (8/20 subjects). The authors have, therefore, adopted lower profile craniocervical instrumentation. A smaller profile generally requires a smaller size and smoother outer contour of the instrumentation. The instrumentation should be configured to allow placement as low as possible over the cranium, to increase the thickness of the tissue overlying the instrumentation.
Concerns about adjacent segment degeneration
The presence of premature disk degeneration and ligamentous laxity with excessive spinal range of motion that characterizes hereditary connective tissue disorders, makes this population vulnerable to both axial and appendicular joint pathology. In most, there had been some degree of instability in the mid-cervical levels before the craniocervical fusion, and many of these subsequently underwent further cervical spine surgery (Table 7). It was surprising, however, that the adjacent segment, C2–3, was rarely the site of isolated segment instability after the craniocervical fusion.
Goel has suggested that ligamentous instability at the craniocervical junction decreases neuromuscular control, leading to further central nervous system injury in a reverberating process that is further exacerbated by the presence of malnutrition and loss of conditioning [120].
Therefore, the putative benefits of craniocervical fusion—improvement of neuromuscular control—should be weighed against the possibility of adjacent segment degeneration and increased proclivity to further spine surgery.
The many co-morbid conditions, the frequent osteopenia, and small bone structure of this population render the appearance of high surgical risk. Yet, surgical outcomes have been surprisingly gratifying, perhaps because this population is younger, and in some respects healthier than those in published studies of craniocervical fusion for rheumatoid arthritis, cancer, trauma, infection, and the elderly [21, 29, 32].
Is kyphosis of the CXA a consideration in the determination to perform a fusion stabilization?
The clivo-axial angle (CXA) has a normal range of 145° to 165°. Flexion of the neck usually decreases the CXA by 10°, and extension of the neck increases the CXA by approximately 10°.
Nagashima reported an angle of less than 130° may produce brainstem compression [62, 73]. Van Gilder reported that a CXA of less than 150° was associated with neurological changes [67]. Kim, Rekate, Klopfenstein, and Sonntag reported that a kyphotic CXA (less than 135°) was a cause of failed Chiari decompression; subsequent open reduction to normalize the CXA resulted insubstantial improvement in 9/10 of the subjects, prompting the authors to describe the kyphotic CXA as a form of “non-traditional basilar invagination” [12]. Morishita suggested that a clivo-axial angle of less than 135° is a risk factor for spinal cord compression [131]. Kubota, in a retrospective series of foramen magnum decompression for Chiari and syringomyelia, reported that the syrinxes failed to abate in those patients in whom the CXA was less than 130° [15].
Brockmeyer, in a retrospective pediatric series of Chiari decompressions, reported that 20% of patients were returned to surgery for reduction and stabilization for kyphotic CXA, craniocervical instability, or the presence of a Chiari 1.5 [4], a finding echoed by Klekamp and others in the adult population [6, 7, 9, 13, 19, 21, 132, 133]. The medulla becomes kinked as the CXA becomes more kyphotic; increasing kyphosis of clivo-axial angle creates a fulcrum by which the odontoid deforms the brainstem [49, 132, 134]. A more complete treatise on the importance of the CXA has been presented elsewhere [70]. In this study, the mean preoperative CXA of 129° was increased to 148° by open reduction of the kyphosis, which correlated with patient improvement. However, the observed improvement may have been the result of craniocervical stabilization.
Pathophysiology
A kyphotic CXA is associated with bending and strain of the lower brainstem and upper spinal cord, and a prelude to neurological deficit [9, 22, 32, 68, 135]. Stretching a neuron nerve decreases neural firing rate and amplitude [136]. The predominant substrate for deformity-induced injury is the axon: electron micrographs show clumping, loss of microtubules and neurofilaments, loss of axon transport and accumulations of axoplasmic material identified as the retraction ball, or retraction bulb, analogous to diffuse axonal injury (DAI) in the brain [137,138,139,140,141,142]. Axon retraction bulbs result from stretch/deformation injury and injury in animal models [98, 100, 143, 144], in the cortico-spinal tracts of the brainstem in infants with shaken baby syndrome, adults with spinal cord injuries, and in basilar invagination [30, 109, 143, 145, 146]. At the molecular level, stretching of nervous tissue deforms Na+ channels, causing increased membrane depolarization and a consequent deleterious influx of Ca++ [147]. The epigenetic effects of mechanical strain are manifest in the observation of increased expression of N-methyl-d-aspartate in the stretched neuron, altered mitochondrial function, and apoptosis [148,149,150].
The clinical improvement observed in this cohort is the presumed consequence of reduction of mechanical deformity of the nervous system and elimination or mitigation of microtrauma from craniocervical instability [10, 16, 49, 107], consistent with the experimental models of axons subjected to strain [149, 151,152,153,154].
Controversy
Treatment of other forms of degenerative and hereditary connective tissue disorders is firmly established in the literature. However, treatment of the EDS patient has been problematic for several reasons. First, though EDS was first described in 1901, the recognition of spinal and neurological manifestations has been only recent [56, 62, 18, 41, 51, 53,54,55, 57,58,59,60,61, 66]. Because this information is new, there is a dearth of evidence upon which to base the management of these genetic disorders.
Second, EDS is considered an “invisible disorder.” EDS patients are characterized by youthful skin, and the appearance of good health, belying their severe pain and disability.
Third, the legion of disparate symptoms due to ligamentous weakness of the joints and spine, and the many co-morbid conditions that accompany EDS, result, understandably, in dismissal by healthcare providers because of the large number of seemingly disparate symptoms.
The authors advocate that the indications for craniocervical fusion should be no different than for the non-EDS population, with the caveat that conditions of ligamentous laxity often require dynamic imaging to demonstrate the pathology [1, 13, 14, 27, 77, 112, 155]. Occiput to C2 bone fusion, as opposed to atlantoaxial fusion in conjunction with fixation, has been discussed [3, 38, 69, 82, 115, 155]. A comparison of various methodologies for bone fusion has also been discussed [83].
The economic significance of hypermobility connective tissue disorders
Treatment of the EDS population is problematic because of the diverse spectrum of disease severity and presentation for whom, in the majority of cases, there is no genetic testing available. In the experience and belief of most EDS care providers, EDS patients suffer through scores of visits to specialists over a mean of 10 years before the diagnosis of EDS is made, during which time they consume vast medical resources through emergency room visits, and unscheduled, often prolonged, admissions to hospital.
The epidemiology of EDS is not known; however, there is little phenotypic difference between patients with h-EDS and the very large population previously diagnosed with joint hypermobility disorder (now referred to as hypermobility spectrum disorder) sharing the same early degeneration of the spine and joints, and the same co-morbid conditions [156,157,158]. Therefore, the authors believe that earlier recognition of these hereditary disorders would substantially reduce costly specialty visits, improve care of this patient population. Early recognition, prudent management, and non-operative therapy may be adequate to stabilize the patient and obviate need of surgery in many cases.
In this cohort, 55% of subjects have returned to work and are paying taxes, or attending school full or part-time with the prospect of future employment, or serving society through caring for their families.
Limitations of the study
This IRB study is a single-center, non-controlled analysis of a small cohort of subjects, referred by medical providers from a broad geographical area (USA and Canada). The study was conceived prior to any surgery, but the subjects were not enrolled until after surgery. Therefore, this should be considered a retrospective study. The outcomes data is to some extent obfuscated by the presence of previous Chiari surgery (five), multiple co-morbid conditions common to EDS, and multiple surgeries within the 5-year follow-up period (12/20). The complexity of the co-morbid conditions and other surgeries are prohibitive to more complex statistical methods. These patients appeared to be the most seriously affected patients within the spectrum of hereditary connective tissue disorders.
Not every patient had cerebellar ectopia (18/20). Subjects may have inaccurately reported the severity of their preoperative symptoms upon questioning at the 2-year follow-up and may have exaggerated the degree of improvement. However, accuracy of reporting was improved through the employment of two independent researchers, who performed the subjects’ interviews at 2 and 5 years. Some subjects may have seen surgery as means validation of their suffering. There was no control for a placebo effect [159].
Conclusion
This study supports the hypothesis that craniocervical reduction, stabilization, and fusion are feasible and associated with clinical improvement in patients in the HCTD population with Chiari malformation or cerebellar ectopia, kyphotic clivo-axial angle, ventral brainstem compression, and/or craniocervical instability. The neurological and functional improvements associated with craniocervical fusion/stabilization appear to be clinically significant and durable. That said, craniocervical fusion should be considered as a last resort after a reasonable course of non-operative treatments.
References
Batzdorf U HF, Rigamonti D. et al (2016) Consensus statement in proceedings of CSF colloquium 2014. In: Batzdorf U (ed) Co-morbidities that complicate the treatment and outcomes of Chiari malformation. Chiari Syringomyelia Foundation, Inc., Lulu, p 3
Bekelis K, Duhaime AC, Missios S, Belden C, Simmons N (2010) Placement of occipital condyle screws for occipitocervical fixation in a pediatric patient with occipitocervical instability after decompression for Chiari malformation. J Neurosurg Pediatr 6:171–176. https://doi.org/10.3171/2010.4.peds09551
Bollo RJ, Riva-Cambrin J, Brockmeyer MM, Brockmeyer DL (2012) Complex Chiari malformations in children: an analysis of preoperative risk factors for occipitocervical fusion. J Neurosurg Pediatr 10:134–141. https://doi.org/10.3171/2012.3.peds11340
Brockmeyer DL (2011) The complex Chiari: issues and management strategies. Neurol Sci 32(Suppl 3):S345–S347. https://doi.org/10.1007/s10072-011-0690-5
Caetano de Barros M, Farias W, Ataide L, Lins S (1968) Basilar impression and Arnold-Chiari malformation. A study of 66 cases. J Neurol Neurosurg Psychiatry 31:596–605
Felbaum D, Spitz S, Sandhu FA (2015) Correction of clivoaxial angle deformity in the setting of suboccipital craniectomy: technical note. J Neurosurg Spine 23:8–15. https://doi.org/10.3171/2014.11.spine14484
Grabb PA, Mapstone TB, Oakes WJ (1999) Ventral brain stem compression in pediatric and young adult patients with Chiari I malformations. Neurosurgery 44:520–527 discussion 527-528
Henderson FC (2016) Cranio-cervical Instability in Patients with Hypermobility Connective Disorders. J Spine
Henderson FC, Wilson WA, Benzel EC (2010) Pathophysiology of cervical myelopathy: biomechanics and deformative stress. Spine Surgery: Techniques, complication avoidance, and management 1
Henderson FC, Wilson WA, Mott S, Mark A, Schmidt K, Berry JK, Vaccaro A, Benzel E (2010) Deformative stress associated with an abnormal clivo-axial angle: a finite element analysis. Surg Neurol Int 1:30. https://doi.org/10.4103/2152-7806.66461
Joseph V, Rajshekhar V (2003) Resolution of syringomyelia and basilar invagination after traction. Case illustration. J Neurosurg 98:298
Kim LJ, Rekate HL, Klopfenstein JD, Sonntag VK (2004) Treatment of basilar invagination associated with Chiari I malformations in the pediatric population: cervical reduction and posterior occipitocervical fusion. J Neurosurg 101:189–195. https://doi.org/10.3171/ped.2004.101.2.0189
Klekamp J (2012) Neurological deterioration after foramen magnum decompression for Chiari malformation type I: old or new pathology? J Neurosurg Pediatr 10:538–547. https://doi.org/10.3171/2012.9.peds12110
Klekamp J (2015) Chiari I malformation with and without basilar invagination: a comparative study. Neurosurg Focus 38:E12. https://doi.org/10.3171/2015.1.focus14783
Kubota M, Yamauchi T, Saeki N Surgical Results of Foramen Magnum Decompression for Chiari Type 1 Malformation associated with Syringomyelia: A Retrospective Study on Neuroradiological Characters influencing Shrinkage of Syringes Y1–2004. - Spinal Surg M1 - Journal Article:- 81
Menezes AH (2012) Craniovertebral junction abnormalities with hindbrain herniation and syringomyelia: regression of syringomyelia after removal of ventral craniovertebral junction compression. J Neurosurg 116:301–309. https://doi.org/10.3171/2011.9.jns11386
Menezes AH, VanGilder JC, Clark CR, el-Khoury G (1985) Odontoid upward migration in rheumatoid arthritis. An analysis of 45 patients with "cranial settling". J Neurosurg 63:500–509. https://doi.org/10.3171/jns.1985.63.4.0500
Milhorat TH, Bolognese PA, Nishikawa M, McDonnell NB, Francomano CA (2007) Syndrome of occipitoatlantoaxial hypermobility, cranial settling, and chiari malformation type I in patients with hereditary disorders of connective tissue. J Neurosurg Spine 7:601–609. https://doi.org/10.3171/spi-07/12/601
Nishikawa M, Ohata K, Baba M, Terakawa Y, Hara M (2004) Chiari I malformation associated with ventral compression and instability: one-stage posterior decompression and fusion with a new instrumentation technique. Neurosurgery 54:1430–1434 discussion 1434-1435
Nishikawa M, Sakamoto H, Hakuba A, Nakanishi N, Inoue Y (1997) Pathogenesis of Chiari malformation: a morphometric study of the posterior cranial fossa. J Neurosurg 86:40–47. https://doi.org/10.3171/jns.1997.86.1.0040
Singh SK, Rickards L, Apfelbaum RI, Hurlbert RJ, Maiman D, Fehlings MG (2003) Occipitocervical reconstruction with the Ohio medical instruments loop: results of a multicenter evaluation in 30 cases. J Neurosurg 98:239–246
Smith JS, Shaffrey CI, Abel MF, Menezes AH (2010) Basilar invagination. Neurosurgery 66:39–47. https://doi.org/10.1227/01.neu.0000365770.10690.6f
Tubbs RS, Beckman J, Naftel RP, Chern JJ, Wellons JC 3rd, Rozzelle CJ, Blount JP, Oakes WJ (2011) Institutional experience with 500 cases of surgically treated pediatric Chiari malformation type I. J Neurosurg Pediatr 7:248–256. https://doi.org/10.3171/2010.12.peds10379
Braca J, Hornyak M, Murali R (2005) Hemifacial spasm in a patient with Marfan syndrome and Chiari I malformation. Case report. J Neurosurg 103:552–554. https://doi.org/10.3171/jns.2005.103.3.0552
Crockard HA, Stevens JM (1995) Craniovertebral junction anomalies in inherited disorders: part of the syndrome or caused by the disorder? Eur J Pediatr 154:504–512
Gabriel KR, Mason DE, Carango P (1990) Occipito-atlantal translation in Down's syndrome. Spine 15:997–1002
Fehlings MG, Cooper P, Errico TJ Rheumatoid arthritis of the cervical spine, Neurosurgical topics: Degenerative disease of the cervical spine Y1–1992. - AANS M1 - Journal Article:- 125–139
Grob D, Schutz U, Plotz G (1999) Occipitocervical fusion in patients with rheumatoid arthritis. Clin Orthop Relat Res 366:46–53
Grob D, Dvorak J, Panjabi MM, Antinnes JA (1994) The role of plate and screw fixation in occipitocervical fusion in rheumatoid arthritis. Spine 19:2545–2551
Henderson FC, Geddes JF, Crockard HA (1993) Neuropathology of the brainstem and spinal cord in end stage rheumatoid arthritis: implications for treatment. Ann Rheum Dis 52:629–637
Ibrahim AG, Crockard HA (2007) Basilar impression and osteogenesis imperfecta: a 21-year retrospective review of outcomes in 20 patients. J Neurosurg Spine 7:594–600. https://doi.org/10.3171/spi-07/12/594
Menezes AH, VanGilder JC (1988) Transoral-transpharyngeal approach to the anterior craniocervical junction. Ten-year experience with 72 patients. J Neurosurg 69:895–903. https://doi.org/10.3171/jns.1988.69.6.0895
Nockels RP, Shaffrey CI, Kanter AS, Azeem S, York JE (2007) Occipitocervical fusion with rigid internal fixation: long-term follow-up data in 69 patients. J Neurosurg Spine 7:117–123. https://doi.org/10.3171/spi-07/08/117
Sandhu FA, Pait TG, Benzel E, Henderson FC (2003) Occipitocervical fusion for rheumatoid arthritis using the inside-outside stabilization technique. Spine 28:414–419. https://doi.org/10.1097/01.brs.0000048460.58471.db
Zygmunt SC, Christensson D, Saveland H, Rydholm U, Alund M (1995) Occipito-cervical fixation in rheumatoid arthritis--an analysis of surgical risk factors in 163 patients. Acta Neurochir 135:25–31
Yoshizumi TMH, Ikenishi Y et al (2014) Occipitocervical fusion with relief of odontoid invagination: atlantoaxial distraction method using cylindrical titanium cage for basilar invagination—case report. Neurosurg Rev 37:519–525
Bick S, Dunn R (2010) Occipito-cervical fusion: review of surgical indications, techniques and clinical outcomes. SA Orthop J 3:26–32
Brockmeyer D (1999) Down syndrome and craniovertebral instability. Topic review and treatment recommendations. Pediatr Neurosurg 31:71–77. https://doi.org/10.1159/000028837
Gordon N (2000) The neurological complications of achondroplasia. Brain Dev 22:3–7
Harkey HL, Crockard HA, Stevens JM, Smith R, Ransford AO (1990) The operative management of basilar impression in osteogenesis imperfecta. Neurosurgery 27:782–786 discussion 786
Henderson FC Sr, Austin C, Benzel E, Bolognese P, Ellenbogen R, Francomano CA, Ireton C, Klinge P, Koby M, Long D, Patel S, Singman EL, Voermans NC (2017) Neurological and spinal manifestations of the Ehlers-Danlos syndromes. Am J Med Genet C: Semin Med Genet 175:195–211. https://doi.org/10.1002/ajmg.c.31549
Jain VK, Mittal P, Banerji D, Behari S, Acharya R, Chhabra DK (1996) Posterior occipitoaxial fusion for atlantoaxial dislocation associated with occipitalized atlas. J Neurosurg 84:559–564. https://doi.org/10.3171/jns.1996.84.4.0559
Keiper GL Jr, Koch B, Crone KR (1999) Achondroplasia and cervicomedullary compression: prospective evaluation and surgical treatment. Pediatr Neurosurg 31:78–83. https://doi.org/10.1159/000028838
Kosnik-Infinger L, Glazier SS, Frankel BM (2014) Occipital condyle to cervical spine fixation in the pediatric population. J Neurosurg Pediatr 13:45–53. https://doi.org/10.3171/2013.9.peds131
Menezes AH (2008) Specific entities affecting the craniocervical region: osteogenesis imperfecta and related osteochondrodysplasias: medical and surgical management of basilar impression. Childs Nerv. Syst. 24:1169–1172. https://doi.org/10.1007/s00381-008-0602-z
Menezes AH, Ryken TC (1992) Craniovertebral abnormalities in Down's syndrome. Pediatr Neurosurg 18:24–33
National Down Syndrome C the position statement. In: “Continuing the Revolution”, 1991
Noske DP, van Royen BJ, Bron JL, Vandertop WP (2006) Basilar impression in osteogenesis imperfecta: can it be treated with halo traction and posterior fusion? Acta Neurochir 148:1301–1305; discussion 1305. https://doi.org/10.1007/s00701-006-0870-x
Sawin PD, Menezes AH (1997) Basilar invagination in osteogenesis imperfecta and related osteochondrodysplasias: medical and surgical management. J Neurosurg 86:950–960. https://doi.org/10.3171/jns.1997.86.6.0950
Tredwell SJ, Newman DE, Lockitch G (1990) Instability of the upper cervical spine in down syndrome. J Pediatr Orthop 10:602–606
Adib N, Davies K, Grahame R, Woo P, Murray KJ (2005) Joint hypermobility syndrome in childhood. A not so benign multisystem disorder? Rheumatology (Oxford, England) 44:744–750. https://doi.org/10.1093/rheumatology/keh557
Castori M, Camerota F, Celletti C, Danese C, Santilli V, Saraceni VM, Grammatico P (2010) Natural history and manifestations of the hypermobility type Ehlers-Danlos syndrome: a pilot study on 21 patients. Am J Med Genet A 152a:556–564. https://doi.org/10.1002/ajmg.a.33231
De Paepe A, Malfait F (2012) The Ehlers-Danlos syndrome, a disorder with many faces. Clin Genet 82:1–11. https://doi.org/10.1111/j.1399-0004.2012.01858.x
Di Palma F, Cronin AH (2005) Ehlers-Danlos syndrome: correlation with headache disorders in a young woman. J Headache Pain 6:474–475. https://doi.org/10.1007/s10194-005-0256-0
Easton V, Bale P, Bacon H, Jerman E, Armon K, Macgregor AJ (2014) The relationship between benign joint hypermobility syndrome and developmental coordination disorders in children. Arthritis Rheumatol 124
el-Shaker M, Watts HG (1991) Acute brachial plexus neuropathy secondary to halo-gravity traction in a patient with Ehlers-Danlos syndrome. Spine 16:385–386
Galan E, Kousseff BG (1995) Peripheral neuropathy in Ehlers-Danlos syndrome. Pediatr Neurol 12:242–245
Halko GJ, Cobb R, Abeles M (1995) Patients with type IV Ehlers-Danlos syndrome may be predisposed to atlantoaxial subluxation. J Rheumatol 22:2152–2155
Jelsma LD, Geuze RH, Klerks MH, Niemeijer AS, Smits-Engelsman BC (2013) The relationship between joint mobility and motor performance in children with and without the diagnosis of developmental coordination disorder. BMC Pediatr 13:35. https://doi.org/10.1186/1471-2431-13-35
Kirby A, Davies R (2007) Developmental coordination disorder and joint hypermobility syndrome--overlapping disorders? Implications for research and clinical practice. Child Care Health Dev 33:513–519. https://doi.org/10.1111/j.1365-2214.2006.00694.x
Milhorat TH, Nishikawa M, Kula RW, Dlugacz YD (2010) Mechanisms of cerebellar tonsil herniation in patients with Chiari malformations as guide to clinical management. Acta Neurochir 152:1117–1127. https://doi.org/10.1007/s00701-010-0636-3
Nagashima C, Tsuji R, Kubota S, Tajima K (1981) [Atlanto-axial, Atlanto-occipital dislocations, developmental cervical canal stenosis in the Ehlers-Danlos syndrome (author's transl)]. No shinkei geka. Neurol Surg 9:601–608
Palmeri S, Mari F, Meloni I, Malandrini A, Ariani F, Villanova M, Pompilio A, Schwarze U, Byers PH, Renieri A (2003) Neurological presentation of Ehlers-Danlos syndrome type IV in a family with parental mosaicism. Clin Genet 63:510–515
Rombaut L, De Paepe A, Malfait F, Cools A, Calders P (2010) Joint position sense and vibratory perception sense in patients with Ehlers-Danlos syndrome type III (hypermobility type). Clin Rheumatol 29:289–295. https://doi.org/10.1007/s10067-009-1320-y
Voermans NC, Drost G, van Kampen A, Gabreels-Festen AA, Lammens M, Hamel BC, Schalkwijk J, van Engelen BG (2006) Recurrent neuropathy associated with Ehlers-Danlos syndrome. J Neurol 253:670–671. https://doi.org/10.1007/s00415-005-0056-0
Voermans NC, van Alfen N, Pillen S, Lammens M, Schalkwijk J, Zwarts MJ, van Rooij IA, Hamel BC, van Engelen BG (2009) Neuromuscular involvement in various types of Ehlers-Danlos syndrome. Ann Neurol 65:687–697. https://doi.org/10.1002/ana.21643
VanGilder JC, Menezes AH, Dolan KD (1987) The craniovertebral junction and its abnormalities. Futura Publishing Company
Breig A (1978) Effects of pincer and clamping actions on the spinal cord Adverse Mechanical Tension in the Central Nervous System:61
Goel A (2004) Treatment of basilar invagination by atlantoaxial joint distraction and direct lateral mass fixation. J Neurosurg Spine 1:281–286. https://doi.org/10.3171/spi.2004.1.3.0281
Henderson FC Sr, Henderson FC Jr, WAt W, Mark AS, Koby M (2018) Utility of the clivo-axial angle in assessing brainstem deformity: pilot study and literature review. Neurosurg Rev 41:149–163. https://doi.org/10.1007/s10143-017-0830-3
Howard RS, Henderson F, Hirsch NP, Stevens JM, Kendall BE, Crockard HA (1994) Respiratory abnormalities due to craniovertebral junction compression in rheumatoid disease. Ann Rheum Dis 53:134–136
Menezes A, Ryken T, Brockmeyer D Abnormalities of the craniocervical junction Y1–2001. - Pediatric Neurosurgery: Surgery of the Developing Nervous System:- 400–422
Nagashima C, Kubota S (1983) Craniocervical abnormalities. Modern diagnosis and a comprehensive surgical approach. Neurosurg Rev 6:187–197
Scoville WB, Sherman IJ (1951) Platybasia, report of 10 cases with comments on familial tendency, a special diagnostic sign, and the end results of operation. Ann Surg 133:496–502
Smoker WR (1994) Craniovertebral junction: normal anatomy, craniometry, and congenital anomalies. Radiographics 14:255–277. https://doi.org/10.1148/radiographics.14.2.8190952
Elements NCD (2016) Clinical research common data Elements (CDEs): radiological metrics standardization for Craniocervical instability. National Institute of Neurological Disorders and Stroke common data element project - approach and methods. Clin Trials 9(33):322–329
Batzdorf U B E, Henderson F. et al (2013) Consensus Statement In Proceedings of CSF Colloquium 2013. In: U B (ed) Basilar Impression & Hypermobility at the Craniocervical Junction. Chiari Syringomyelia Foundation, Lulu,
Harris JH Jr, Carson GC, Wagner LK (1994) Radiologic diagnosis of traumatic occipitovertebral dissociation: 1. Normal occipitovertebral relationships on lateral radiographs of supine subjects. AJR Am J Roentgenol 162:881–886. https://doi.org/10.2214/ajr.162.4.8141012
Elements NCD Clinical Research Common Data Elements (CDEs): Radiological Metrics Standardization for Craniocervical Instability Y1–2016
Aryan HE, Newman CB, Nottmeier EW, Acosta FL Jr, Wang VY, Ames CP (2008) Stabilization of the atlantoaxial complex via C-1 lateral mass and C-2 pedicle screw fixation in a multicenter clinical experience in 102 patients: modification of the harms and Goel techniques. J Neurosurg Spine 8:222–229. https://doi.org/10.3171/spi/2008/8/3/222
Dickman CA, Sonntag VK (1998) Posterior C1-C2 transarticular screw fixation for atlantoaxial arthrodesis. Neurosurgery 43:275–280 discussion 280-271
Goel A, Bhatjiwale M, Desai K (1998) Basilar invagination: a study based on 190 surgically treated patients. J Neurosurg 88:962–968. https://doi.org/10.3171/jns.1998.88.6.0962
Sawin PD, Traynelis VC, Menezes AH (1998) A comparative analysis of fusion rates and donor-site morbidity for autogeneic rib and iliac crest bone grafts in posterior cervical fusions. J Neurosurg 88:255–265. https://doi.org/10.3171/jns.1998.88.2.0255
Malfait F, Francomano C, Byers P, Belmont J, Berglund B, Black J, Bloom L, Bown JM et al (2017) The 2017 international classification of the Ehlers–Danlos syndromes. Am J Med Genet 175:8–26. https://doi.org/10.1002/ajmg.c.31552
Tinkle B, Castori M, Berglund B, Cohen H, Grahame R, Kazkaz H, Levy H (2017) Hypermobile Ehlers–Danlos syndrome (a.k.a. Ehlers–Danlos syndrome type III and Ehlers–Danlos syndrome hypermobility type): clinical description and natural history. Am J Med Genet 175C:48–69. https://doi.org/10.1002/ajmg.c.31538
Byers PH (2001) Folding defects in fibrillar collagens. Philos Trans R Soc.B 356:151–158. https://doi.org/10.1098/rstb.2000.0760
Malfait F, Coucke P, Symoens S, Loeys B, Nuytinck L, De Paepe A (2005) The molecular basis of classic Ehlers-Danlos syndrome: a comprehensive study of biochemical and molecular findings in 48 unrelated patients. Hum Mutat 25:28–37
Steinmann B, Royce PM, Superti-Furga A The Ehlers-Danlos Syndrome Y1–2003. - Connective Tissue and Its Heritable Disorders: Molecular, Genetic, and Medical Aspects:- 431
Castori M, Voermans NC (2014) Neurological manifestations of Ehlers-Danlos syndrome(s): a review. Iran J Neurol 13:190–208
Savasta S, Merli P, Ruggieri M, Bianchi L, Sparta MV (2011) Ehlers-Danlos syndrome and neurological features: a review. Childs Nerv Syst 27:365–371. https://doi.org/10.1007/s00381-010-1256-1
Martin MD, Bruner HJ, Maiman DJ (2010) Anatomic and biomechanical considerations of the craniovertebral junction. Neurosurgery 66:2–6. https://doi.org/10.1227/01.neu.0000365830.10052.87
Tubbs RS, Hallock JD, Radcliff V, Naftel RP, Mortazavi M, Shoja MM, Loukas M, Cohen-Gadol AA (2011) Ligaments of the craniocervical junction. J Neurosurg Spine 14:697–709. https://doi.org/10.3171/2011.1.spine10612
Koby M (2016) The discordant report - pathological radiological findings: A peripatetic review of salient features of neuropathology in the setting of an erstwhile standard 'normal' radiological assessment. In: Batzdorf U (ed) Co-Morbidities that Complicate the Treatment and Outcomes of Chiari Malformation. Chiari Syringomyelia Foundation Inc., Lulu, p 50
Steinmetz MP, Mroz TE, Benzel EC (2010) Craniovertebral junction: biomechanical considerations. Neurosurgery 66:7–12. https://doi.org/10.1227/01.neu.0000366109.85796.42
Dvorak J, Hayek J, Zehnder R (1987) CT-functional diagnostics of the rotatory instability of the upper cervical spine. Part 2. An evaluation on healthy adults and patients with suspected instability. Spine 12:726–731
Tubbs RS, Stetler W, Shoja MM, Loukas M, Hansasuta A, Liechty P, Acakpo-Satchivi L, Wellons JC, Blount JP, Salter EG, Oakes WJ (2007) The lateral atlantooccipital ligament. Surg Radiol Anat 29:219–223. https://doi.org/10.1007/s00276-007-0196-2
Geddes JF, Hackshaw AK, Vowles GH, Nickols CD, Whitwell HL (2001) Neuropathology of inflicted head injury in children. I. Patterns of brain damage. Brain 124:1290–1298
Hardman JM (1979) The pathology of traumatic brain injuries. Adv Neurol 22:15–50
Lindenberg R, Freytag E (1970) Brainstem lesions characteristic of traumatic hyperextension of the head. Arch Pathol 90:509–515
Riggs JE, Schochet SS Jr (1995) Spastic quadriparesis, dysarthria, and dysphagia following cervical hyperextension: a traumatic pontomedullary syndrome. Mil Med 160:94–95
Nash L, Nicholson H, Lee AS, Johnson GM, Zhang M (2005) Configuration of the connective tissue in the posterior atlanto-occipital interspace: a sheet plastination and confocal microscopy study. Spine 30:1359–1366
Milhorat TH, Chou MW, Trinidad EM, Kula RW, Mandell M, Wolpert C, Speer MC (1999) Chiari I malformation redefined: clinical and radiographic findings for 364 symptomatic patients. Neurosurgery 44:1005–1017
Pang D, Thompson DN (2011) Embryology and bony malformations of the craniovertebral junction. Childs Nerv Syst 27:523–564. https://doi.org/10.1007/s00381-010-1358-9
Thakar S, Sivaraju L, Jacob KS, Arun AA, Aryan S, Mohan D, Sai Kiran NA, Hegde AS (2018) A points-based algorithm for prognosticating clinical outcome of Chiari malformation type I with syringomyelia: results from a predictive model analysis of 82 surgically managed adult patients. J Neurosurg Spine 28:23–32. https://doi.org/10.3171/2017.5.spine17264
Celletti C, Galli M, Cimolin V, Castori M, Albertini G, Camerota F (2012) Relationship between fatigue and gait abnormality in joint hypermobility syndrome/Ehlers-Danlos syndrome hypermobility type. Res Dev Disabil 33:1914–1918. https://doi.org/10.1016/j.ridd.2012.06.018
Dyste GN, Menezes AH, VanGilder JC (1989) Symptomatic Chiari malformations. An analysis of presentation, management, and long-term outcome. J Neurosurg 71:159–168. https://doi.org/10.3171/jns.1989.71.2.0159
Goel A, Shah A (2009) Reversal of longstanding musculoskeletal changes in basilar invagination after surgical decompression and stabilization. J Neurosurg Spine 10:220–227. https://doi.org/10.3171/2008.12.spine08499
da Silva JA, Brito JC, da Nobrega PV (1992) Autonomic nervous system disorders in 230 cases of basilar impression and Arnold-Chiari deformity. Neurochirurgia 35:183–188
Henderson FC, Geddes JF, Vaccaro AR, Woodard E, Berry KJ, Benzel EC (2005) Stretch-associated injury in cervical spondylotic myelopathy: new concept and review. Neurosurgery 56:1101–1113 discussion 1101-1113
Fielding JW (1957) Cineroentgenography of the normal cervical spine. J Bone Joint Surg Am 39:1280–1288
Werne S (1957) Studies in spontaneous atlas dislocation. Acta Orthop Scand Suppl 23:1–150
White AA, Panjabi MM Clinical biomechanics of the spine 2nd edition Y1–1990
Wiesel SW, Rothman RH (1979) Occipitoatlantal hypermobility. Spine 4:187–191
Wolfla CE (2006) Anatomical, biomechanical, and practical considerations in posterior occipitocervical instrumentation. The spine journal : official journal of the North American Spine Society 6:225s–232s. https://doi.org/10.1016/j.spinee.2006.09.001
Dickman CA, Douglas RA, Sonntag VH (1990) Occipitocervical fusion: posterior stabilization of the craniovertebral junction and upper cervical spine. BNI Quarterly 6:2–14
Batzdorf U, Henderson FC, Rigamonti D (2016) Co-Morbidities that Complicate the Treatment and Outcomes of Chiari Malformation. Proceedings of the CSF Colloquium 2014. Chiari Syringomyelia Foundation, Inc., Lulu
Menezes A (1995) Primary Craniovertebral anomalies and the hindbrain herniation syndrome (Chiari I): Data Base analysis. Pediatr Neursurg 23:260–269. https://doi.org/10.1159/000120969
Jacome DE (1999) Headache in Ehlers-Danlos syndrome. Cephalalgia 19:791–796. https://doi.org/10.1046/j.1468-2982.1999.1909791.x
Satti SR, Leishangthem L, Chaudry MI (2015) Meta-analysis of CSF diversion procedures and Dural venous sinus stenting in the setting of medically refractory idiopathic intracranial hypertension. AJNR Am J Neuroradiol 36:1899–1904. https://doi.org/10.3174/ajnr.A4377
Goel A (2012) Instability and basilar invagination. Journal of craniovertebral junction & spine 3:1–2. https://doi.org/10.4103/0974-8237.110115
Izeki M, Neo M, Takemoto M, Fujibayashi S, Ito H, Nagai K, Matsuda S (2014) The O-C2 angle established at occipito-cervical fusion dictates the patient's destiny in terms of postoperative dyspnea and/or dysphagia. Eur Spine J 23:328–336. https://doi.org/10.1007/s00586-013-2963-6
Breig A (1970) Overstretching of and circumscribed pathological tension in the spinal cord—a basic cause of symptoms in cord disorders. J Biomech Eng 3:7–9
Bendik EM, Tinkle BT, Al-shuik E, Levin L, Martin A, Thaler R, Atzinger CL, Rueger J, Martin VT (2011) Joint hypermobility syndrome: a common clinical disorder associated with migraine in women. Cephalalgia 31:603–613. https://doi.org/10.1177/0333102410392606
Bulbena A, Baeza-Velasco C, Bulbena-Cabré A, Pailhez G, Critchley H, Chopra P, Mallorquí-Bagué N, Frank C, Porges S (2017) Psychiatric and psychological aspects in the Ehlers–Danlos syndromes. Am J Med Genet 175:237–245
Bulbena A, Pailhez G, Bulbena-Cabré A, Mallorquí-Bagué N, Baeza-Velasco C (2015) Joint hypermobility, anxiety and psychosomatics: two and a half decades of progress toward a new phenotype. Clinical Challenges in the Biopsychosocial Interface (Karger Publishers) 34:143–157
Castori M, Morlino S, Ghibellini G, Celletti C, Camerota F, Grammatico P (2015) Connective tissue, Ehlers-Danlos syndrome(s), and head and cervical pain. American journal of medical genetics part C, seminars in medical. genetics 169c:84–96. https://doi.org/10.1002/ajmg.c.31426
Farb RI, Vanek I, Scott JN, Mikulis DJ, Willinsky RA, Tomlinson G, terBrugge KG (2003) Idiopathic intracranial hypertension: the prevalence and morphology of sinovenous stenosis. Neurology 60:1418–1424
Hamonet C, Ducret L, Marié-Tanay C, Brock I (2016) Dystonia in the joint hypermobility syndrome (aka Ehlers-Danlos syndrome, hypermobility type). SOJ Neurol 3:1–3
Tinkle BT (2014) Joint hypermobility and headache. Headache 54:1412–1413. https://doi.org/10.1111/head.12416
Sasso RC, Jeanneret B, Fischer K, Magerl F (1994) Occipitocervical fusion with posterior plate and screw instrumentation. A long-term follow-up study. Spine 19:2364–2368
Morishita YFJ, Naito M, Hymanson HJ, Taghavi C, Wang JC (2009) The kinematic relationships of the upper cervical spine. Spine 34:2642–2645
Klimo P Jr, Kan P, Rao G, Apfelbaum R, Brockmeyer D (2008) Os odontoideum: presentation, diagnosis, and treatment in a series of 78 patients. Journal of neurosurgery Spine 9:332–342. https://doi.org/10.3171/spi.2008.9.10.332
Menezes A (2014) Clival and Craniovertebral junction Chordomas. World Neurosurg 81:690–692. https://doi.org/10.1016/j.wneu.2013.03.050
Tubbs RS, McGirt MJ, Oakes WJ (2003) Surgical experience in 130 pediatric patients with Chiari I malformations. J Neurosurg 99:291–296. https://doi.org/10.3171/jns.2003.99.2.0291
Breig A (1989) Skull traction and cervical cord injury: a new approach to improved rehabilitation. Springer-Verlag, New York
Shi R, Whitebone J (2006) Conduction deficits and membrane disruption of spinal cord axons as a function of magnitude and rate of strain. J Neurophysiol 95:3384–3390
Gennarelli TA (1997) The pathobiology of traumatic brain injury. Neuroscientist 3:73–81. https://doi.org/10.1177/107385849700300117
Jafari SS, Maxwell WL, Neilson M, Graham DI (1997) Axonal cytoskeletal changes after non-disruptive axonal injury. J Neurocytol 26:207–221
Maxwell WL, Domleo A, McColl G, Jafari SS, Graham DI (2003) Post-acute alterations in the axonal cytoskeleton after traumatic axonal injury. J Neurotrauma 20:151–168. https://doi.org/10.1089/08977150360547071
Maxwell WL, Islam MN, Graham DI, Gennarelli TA (1994) A qualitative and quantitative analysis of the response of the retinal ganglion cell soma after stretch injury to the adult Guinea-pig optic nerve. J Neurocytol 23:379–392
Maxwell WL, Kosanlavit R, McCreath BJ, Reid O, Graham DI (1999) Freeze-fracture and cytochemical evidence for structural and functional alteration in the axolemma and myelin sheath of adult Guinea pig optic nerve fibers after stretch injury. J Neurotrauma 16:273–284. https://doi.org/10.1089/neu.1999.16.273
Povlishock JT (1992) Traumatically induced axonal injury: pathogenesis and pathobiological implications. Brain Pathol (Zurich, Switzerland) 2:1–12
Chung RS, Staal JA, McCormack GH, Dickson TC, Cozens MA, Chuckowree JA, Quilty MC, Vickers JC (2005) Mild axonal stretch injury in vitro induces a progressive series of neurofilament alterations ultimately leading to delayed axotomy. J Neurotrauma 22:1081–1091. https://doi.org/10.1089/neu.2005.22.1081
Saatman KE, Abai B, Grosvenor A, Vorwerk CK, Smith DH, Meaney DF (2003) Traumatic axonal injury results in biphasic calpain activation and retrograde transport impairment in mice. J Cereb Blood Flow Metab 23:34–42. https://doi.org/10.1097/01.wcb.0000035040.10031.b0
Bunge RP, Puckett WR, Becerra JL, Marcillo A, Quencer RM (1993) Observations on the pathology of human spinal cord injury. A review and classification of 22 new cases with details from a case of chronic cord compression with extensive focal demyelination. Adv Neurol 59:75–89
Geddes JF, Whitwell HL, Graham DI (2000) Traumatic axonal injury: practical issues for diagnosis in medicolegal cases. Neuropathol Appl Neurobiol 26:105–116
Wolf JA, Stys PK, Lusardi T, Meaney D, Smith DH (2001) Traumatic axonal injury induces calcium influx modulated by tetrodotoxin-sensitive sodium channels. J Neurosci 21:1923–1930
Arundine M, Aarts M, Lau A, Tymianski M (2004) Vulnerability of central neurons to secondary insults after in vitro mechanical stretch. J Neurosci 24:8106–8123. https://doi.org/10.1523/jneurosci.1362-04.2004
Li GL, Brodin G, Farooque M, Funa K, Holtz A, Wang WL, Olsson Y (1996) Apoptosis and expression of Bcl-2 after compression trauma to rat spinal cord. J Neuropathol Exp Neurol 55:280–289
Liu XZ, Xu XM, Hu R, Du C, Zhang SX, McDonald JW, Dong HX, Wu YJ, Fan GS, Jacquin MF, Hsu CY, Choi DW (1997) Neuronal and glial apoptosis after traumatic spinal cord injury. J Neurosci 17:5395–5406
Galbraith JA, Thibault LE, Matteson DR (1993) Mechanical and electrical responses of the squid giant axon to simple elongation. J Biomech Eng 115:13–22
Povlishock JT, Jenkins LW (1995) Are the pathobiological changes evoked by traumatic brain injury immediate and irreversible? Brain Pathol (Zurich, Switzerland) 5:415–426
Shi R, Pryor JD (2002) Pathological changes of isolated spinal cord axons in response to mechanical stretch. Neuroscience 110:765–777
Torg JS, Thibault L, Sennett B, Pavlov H (1995) The Nicolas Andry award. The pathomechanics and pathophysiology of cervical spinal cord injury. Clin Orthop Relat Res:259–269
Botelho RVNE, Patriota GC, Daniel JW, Dumont PA, Rotta JM (2007) Basilar invagination: craniocervical instability treated with cervical traction and occipitocervical fixation. J Neurosurg Spine 7:444–449
Grahame R, Bird HA, Child A (2000) The revised (Brighton 1998) criteria for the diagnosis of benign joint hypermobility syndrome (BJHS). J Rheumatol 27:1777–1779
Sacheti A, Szemere J, Bernstein B, Tafas T, Schechter N, Tsipouras P (1997) Chronic pain is a manifestation of the Ehlers-Danlos syndrome. J Pain Symptom Manag 14:88–93
Tinkle BT, Bird HA, Grahame R, Lavallee M, Levy HP, Sillence D (2009) The lack of clinical distinction between the hypermobility type of Ehlers-Danlos syndrome and the joint hypermobility syndrome (a.k.a. hypermobility syndrome). Am J Med Genet A 149a:2368–2370. https://doi.org/10.1002/ajmg.a.33070
Wartolowska K, Judge A, Hopewell S, Collins GS, Dean BJ, Rombach I, Brindley D, Savulescu J, Beard DJ, Carr AJ (2014) Use of placebo controls in the evaluation of surgery: systematic review. BMJ (Clinical research ed) 348:g3253. https://doi.org/10.1136/bmj.g3253
Acknowledgements
To Betsy G. Henderson for help with layout and editing, and to the patients who have informed this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was solely funded by the Metropolitan Neurosurgery Group, LLC.
Conflict of interest
One of the senior authors (FCH Sr.) was first author of a patent for a craniocervical stabilization device, which is currently being developed by LifeSpine, Inc. (Huntley, IL) and related patents discussing finite element analysis (spinal cord stress injury analysis) of the central nervous system, mathematical prediction of neurobehavioral change, and related devices pertinent to disorders of the craniocervical junction. The same author (FCH Sr) has donated his royalties for the craniocervical device to the Chiari Syringomyelia Foundation (CSF).
Ethical approval
The study was conducted under the auspices of the Greater Baltimore Medical Center (IRB# 10-036-06: GBMC). This study followed all requirements of the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Every patient signed an informed consent form to participate in the study.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Henderson, F.C., Francomano, C.A., Koby, M. et al. Cervical medullary syndrome secondary to craniocervical instability and ventral brainstem compression in hereditary hypermobility connective tissue disorders: 5-year follow-up after craniocervical reduction, fusion, and stabilization. Neurosurg Rev 42, 915–936 (2019). https://doi.org/10.1007/s10143-018-01070-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-018-01070-4