Abstract
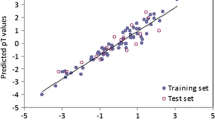
Quantitative structure-toxicity relationship (QSTR) studies have proved to be a valuable approach in research on the toxicity of organic chemicals for ranking chemical substances with respect to their potential hazardous effects on living systems. With this background, we have modeled here the acute lethal toxicity of 51 benzene derivatives with recently introduced extended topochemical atom (ETA) indices [Roy and Ghosh, Internet Electron J Mol Des 2:599–620 (2003)]. We also compared the ETA relations with non-ETA models derived from different topological indices (Wiener W, Balaban J, flexibility index ϕ, Hosoya Z, Zagreb, molecular connectivity indices, E-state indices and kappa shape indices) and physicochemical parameters (AlogP98, MolRef,H_bond_donor and H_bond_acceptor). Genetic function approximation (GFA) and factor analysis (FA) were used as the data-preprocessing steps for the development of final multiple linear regression (MLR) equations. Principal-component regression analysis (PCRA) was also used to extract the total information from the ETA/non-ETA/combined matrices. All the models developed were cross-validated using leave-one-out (LOO) and leave-many-out techniques. The summary of the statistics of the best models is as follows: (1) FA-MLR: ETA model- Q 2 (LOO)=0.852, R 2=0.894; non-ETA model- Q 2=0.782, R 2=0.835; ETA + non-ETA model-Q 2 =0.815, R 2=0.859. (2) GFA-MLR: ETA model-Q 2 =0.847, R 2=0.915; non-ETA model-Q 2 =0.863, R 2=0.898; ETA + non-ETA model-Q 2 =0.859, R 2=0.893. 3. PCRA: ETA model-Q 2 =0.864, R 2=0.901; non-ETA model- Q 2=0.866, R 2=0.922; ETA + non-ETA model-Q 2=0.846, R 2=0.890. The statistical quality of the ETA models is comparable to that of non-ETA models. Again, use of non-ETA descriptors in addition to ETA descriptors does not increase the statistical acceptance of the relations significantly. The predictive potential of these models was better than that of the previously reported models using physicochemical parameters [Huang et al., Chemosphere 53:963–970 (2003)]. The relations from ETA descriptors suggest a parabolic dependence of the toxicity on molecular size. Furthermore, the toxicity increases with functionality contribution of chloro substituent and decreases with those of methoxy, hydroxy, carboxy and amino groups. This study suggests that ETA parameters are sufficiently rich in chemical information to encode the structural features that contribute significantly to the acute toxicity of benzene derivatives to Rana japonica.
Similar content being viewed by others
References
Kavlock RJ, Daston GP, Derosa C, Fenner-Crisp P, Gray LE, Kaattari S, Lucier G, Luster M, Mac MJ, Maczka C, Miller R, Moore J, Rolland R, Csott G, Sheehan DM, Sinks T, Tilson HA (1996) Environ Health Perspect 104:715–740
Sanderson H, Jonson DJ, Reitsma T, Brain RA, Wilson CJ, Solomon KR (2004) Regul Toxicol Pharmacol 39:158–183
McKinney JD, Richard A, Waller C, Newman MC, Gerberick F (2000) Toxicol Sci 56:8–17
Comber MH, Walker JD, Watts C, Hermens J (2003) Environ Toxicol Chem 22:1822–1828
Russom CL, Anderson EB, Greenwood BE, Pilli A (1991) Sci Total Environ 109–110:667–670
Ren S (2002) Environ Toxicol 17:119–127
Rose K, Hall LH (2003) SAR QSAR Environ Res 14:113–129
Mazzatorta P, Benfenati E, Neagu CD, Gini G (2003) J Chem Inf Comput Sci 43:513–518
Vighi M, Gramatica P, Consolaro F, Todeschini R (2001) Ecotoxicol Environ Saf 49:206–220
Bask SC, Grunwald GD, Gute BD, Balasubramanian K, Opitz D (2000) J Chem Inf Comput Sci 40:885–890
Devillers J (2001) SAR QSAR Environ Res 11:397–417
Roy K, Ghosh G (2003) Internet Electron J Mol Des 2:599–620; http://www.biochempress.com
Roy K, Ghosh G (2004) J Chem Inf Comput Sci 44:559–567
Roy K, Ghosh G (2004) QSAR Comb Sci 23:99–108
Roy K, Ghosh G (2004) QSAR Comb Sci 23:526–535
Roy K, Ghosh G (2005) Bioorg Med Chem 13:1185–1194
Pal DK, Sengupta C, De AU (1988) Indian J Chem 27B:734–739
Pal DK, Sengupta C, De AU (1989) Indian J Chem 28B:261–267
Pal DK, Sengupta M, Sengupta C, De AU (1990) Indian J Chem 29B:451–454
Pal DK, Purkayastha SK, Sengupta C, De AU (1992) Indian J Chem 31B:109–114
Roy K, Pal DK, De AU, Sengupta C (1999) Indian J Chem 38B:664–671
Roy K, Pal DK, De AU, Sengupta C (2001) Indian J Chem 40B:129–135
Roy K, Saha A (2003) J Mol Model 9:259–270
Roy K, Saha A (2003) Internet Electron J Mol Des 2:288–305; http://www.biochempress.com
Roy K, Saha A (2003) Internet Electron J Mol Des 2:475–491 http://www.biochempress.com
Roy K, Chakroborty S, Ghosh CC, Saha A (2004) J Indian Chem Soc 81:115–125
Huang, H, Wang X, Ou W, Zhao J, Shao Y, Wang L (2003) Chemosphere 53:963–970
Rogers D, Hopfinger AJ (1994) J Chem Inf Comput Sci 34:854–866
Fan Y, Shi LM, Kohn KW, Pommier Y, Weinstein JN (2001) J Med Chem 44:3254–3263
Lewi PJ (1980) Multivariate data analysis in structure-activity relationships. In: Ariens EJ (ed) Drug design, vol 10. Academic Press, NY, pp 307–342
Franke R, Gruska A (1995) Principal component and factor analysis. In: van de Waterbeemd H (ed) Chemometric methods in molecular design, vol 2. VCH, Weinheim, pp 113–163
Cerius 2 Version 4.8 is a product of Accelrys Inc., San Diego, CA
SPSS is statistical software of SPSS Inc., IL
The GW-BASIC programs RRR98, KRETA1, KRETA2, KRPRES1 and KRPRES2 were developed by Kunal Roy and standardized using known data sets
Snedecor GW, Cochran WG (1967) Statistical methods. Oxford and IBH Publishing Co Pvt Ltd, New Delhi, pp 381–418
Wold S, Eriksson L (1995) Statistical validation of QSAR results. In: van de Waterbeemd H (ed) Chemometric methods in molecular design. VCH, Weinheim, pp 312–317
Debnath AK (2001) Quantitative structure-activity relationship (QSAR): A versatile tool in drug design. In: Ghose AK, Viswanadhan VN (eds) Combinatorial library design and evaluation. Marcel Dekker, NY, pp 73–129
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roy, K., Ghosh, G. QSTR with extended topochemical atom (ETA) indices. VI. Acute toxicity of benzene derivatives to tadpoles (Rana japonica). J Mol Model 12, 306–316 (2006). https://doi.org/10.1007/s00894-005-0033-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-005-0033-7