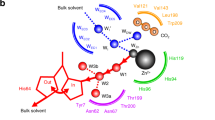
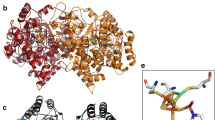
Abstract
Eight Ni proteins are known and three of these, CO dehydrogenase (CODH), acetyl-CoA synthase (ACS), and hydrogenase, are Ni-Fe-S proteins. In the last three years, the long-awaited structures of CODH and ACS have been solved. The bioinorganic community was shocked, as the structures of the active sites of CODH and ACS, the C- and A-cluster, respectively, which each had been predicted to consist of a [Fe4S4] cluster bridged to a single Ni, revealed unexpected compositions and arrangements. Crystal structures of ACS revealed major differences in protein conformation and in A-cluster composition; for example, a [Fe4S4] cluster bridged to a binuclear center in which one of the metal binding sites was occupied by Ni, Cu, or Zn. Recent studies have revealed Ni-Ni to be the active state, unveiled the source of the heterogeneity that had plagued studies of CODH/ACS for decades, and produced a metal-replacement strategy to generate highly active and nearly homogeneous enzyme.




Similar content being viewed by others
Abbreviations
- CFeSP:
-
corrinoid iron-sulfur protein
- CH3H4folate:
-
methyltetrahydrofolate
- CODH/ACS:
-
carbon monoxide dehydrogenase/acetyl-CoA synthases
- ENDOR:
-
electron nuclear double resonance
- MeTr:
-
methyltransferase
References
Drennan CL, Heo J, Sintchak MD, Schreiter E, Ludden PW (2001) Proc Natl Acad Sci USA 98:11973–11978
Dobbek H, Svetlitchnyi V, Gremer L, Huber R, Meyer O (2001) Science 293:1281–1285
Doukov T, Iverson TM, Seravalli J, Ragsdale SW, Drennan CL (2002) Science 298:567–572
Darnault C, Volbeda A, Kim EJ, Legrand P, Vernède X, Lindahl PA, Fontecilla-Camps JC (2003) Nat Struct Biol 10:271–279
Svetlitchnyi V, Peschel C, Acker G, Meyer O (2001) J Bacteriol 183:5134–5144
Maynard EL, Lindahl PA (2001) Biochemistry 40:13262–13267
Seravalli J, Ragsdale SW (2000) Biochemistry 39:1274–1277
Maynard EL, Lindahl PA (1999) J Am Chem Soc 121:9221–9222
Svetlichnyi V, Dobbek H, Meyer-Klaucke W, Meins T, Thiele B, Römer P, Huber R, Meyer O (2004) Proc Natl Acad Sci USA 101:446–451
Huang W, Jia J, Cummings J, Nelson M, Schneider G, Lindqvist Y (1997) Structure 5:691–699
Nagashima S, Nakasako M, Dohmae N, Tsujimura M, Takio K, Odaka M, Yohda M, Kamiya N, Endo I (1998) Nat Struct Biol 5:347–351
Nicolet Y, Lemon BJ, Fontecilla-Camps JC, Peters JW (2000) Trends Biochem Sci 25:138–143
Bramlett MR, Tan X, Lindahl PA (2003) J Am Chem Soc 125:9316–9317
Seravalli J, Gu W, Tam A, Strauss E, Begley TP, Cramer SP, Ragsdale SW (2003) Proc Natl Acad Sci USA 100:3689–3694
Gencic S, Grahame DA (2003) J Biol Chem 278:6101–6110
Seravalli J, Xiao Y, Gu W, Cramer SP, Antholine WE, Krymov V, Gerfen GJ, Ragsdale SW (2004) Biochemistry 43:3944–3955
Shin W, Anderson ME, Lindahl PA (1993) J Am Chem Soc 115:5522–5526
Schenker RP, Brunold TC (2003) J Am Chem Soc 125:13962–13963
Barondeau DP, Lindahl PA (1997) J Am Chem Soc 119:3959–3970
Seravalli J, Kumar M, Ragsdale SW (2002) Biochemistry 41:1807–1819
Rae TD, Schmidt PJ, Pufahl RA, Culotta VC, O’Halloran TV (1999) Science 284:805–808
Ensign SA, Ludden PW (1991) J Biol Chem 266:18395–18403
Acknowledgements
The authors gratefully acknowledge GM69857 for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Drennan, C.L., Doukov, T.I. & Ragsdale, S.W. The metalloclusters of carbon monoxide dehydrogenase/acetyl-CoA synthase: a story in pictures. J Biol Inorg Chem 9, 511–515 (2004). https://doi.org/10.1007/s00775-004-0563-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-004-0563-y