Abstract.
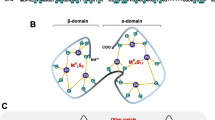
Metallothionein-3 (MT-3), also known as neuronal growth inhibitory factor, is a metalloprotein expressed almost exclusively in the brain. Isolated MT-3 contains four Cu(I) and three Zn(II) ions organized in homometallic metal-thiolate clusters located in two independent protein domains. In this work a Cu(I) binding to metal-free MT-3 has been studied, aiming at the better understanding of the domain specificity for this metal ion. The cluster formation was followed by electronic absorption, circular dichroism, and by luminescence spectroscopy at room temperature and 77 K. The stepwise incorporation of Cu(I) into recombinant human apo-MT-3 revealed the cooperative formation of two Cu4S9 clusters in succession, formed in both protein domains, i.e. Cu4- and Cu8-MT-3. Further binding of four Cu(I) caused an expansion of these Cu(I) cores, leading to fully metal-loaded Cu12-MT-3 containing Cu6S9 and Cu6S11 clusters in the β- and α-domains of the protein, respectively. The location of the preferentially formed Cu4 cluster in the protein was established by immunochemistry. Using domain-specific antibodies, in combination with limited tryptic digestion of a partially metal-occupied Cu4-MT-3, we could demonstrate that the Cu4S9 cluster is located in the N-terminal β-domain of the protein that contains a total of nine cysteine ligands. Electronic supplementary material to this paper, comprising Table S1 (amino acid sequences of peptides used in immunization) and Fig. S1 (luminescence spectra of Cu(I) titrated apo-MT-3), can be obtained by using the Springer Link server located at http://dx.doi.org/10.1007/s00775-002-0339-1.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Electronic Publication
Rights and permissions
About this article
Cite this article
Roschitzki, B., Vašák, M. A distinct Cu4-thiolate cluster of human metallothionein-3 is located in the N-terminal domain. J Biol Inorg Chem 7, 611–616 (2002). https://doi.org/10.1007/s00775-002-0339-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-002-0339-1