Abstract
Inonotus obliquus (Fr.) Pilat is a white rot fungus belonging to the family Hymenochaetaceae in the Basidiomycota. In nature, this fungus rarely forms a fruiting body but usually an irregular shape of sclerotial conk called ‘Chaga’. Characteristically, I. obliquus produces massive melanins released to the surface of Chaga. As early as in the sixteenth century, Chaga was used as an effective folk medicine in Russia and Northern Europe to treat several human malicious tumors and other diseases in the absence of any unacceptable toxic side effects. Chemical investigations show that I. obliquus produces a diverse range of secondary metabolites including phenolic compounds, melanins, and lanostane-type triterpenoids. Among these are the active components for antioxidant, antitumoral, and antiviral activities and for improving human immunity against infection of pathogenic microbes. Geographically, however, this fungus is restricted to very cold habitats and grows very slowly, suggesting that Chaga is not a reliable source of these bioactive compounds. Attempts for culturing this fungus axenically all resulted in a reduced production of bioactive metabolites. This review examines the current progress in the discovery of chemical diversity of Chaga and their biological activities and the strategies to modulate the expression of desired pathways to diversify and up-regulate the production of bioactive metabolites by the fungus grown in submerged cultures for possible drug discovery.
Similar content being viewed by others
Introduction
Inonotus obliquus (Fr.) Pilat is a white rot fungus belonging to the family Hymenochaetaceae in the Basidiomycota, inhabiting primarily the trunks of Betula trees but also occasionally on other angiosperm trees in nature (Ryvarden and Gilbertson 1993). This fungus rarely forms the fertile fruiting body but usually an irregular shape of sclerotial conk with the appearance of burnt charcoal, which is termed ‘Chaga’ in Russia, Northern Europe, and most of Baltic countries. Morphologically, Chaga is shaped like a wedge, bursts through the bark, and appears as large gall-like structures, varying in size from 5 to 40 cm in diameter, with a very irregularly cracked and deeply fissured surface. Inside the burnt charcoal surface is rusty-colored woody texture consisting of interwoven mycelia. Geographically, I. obliquus is distributed in Russia, Korea, Eastern Europe, Northern China, Northern areas of the United States, and the North Carolina mountains (Huang 2002). Early in the sixteenth century, Chaga had been used as an effective folk medicine in Russia and Northern Europe to treat cancer, gastritis, ulcers, and tuberculosis (Kim et al. 2007b) in the absence of any unacceptable toxic side effects (Zheng et al. 2009d). Traditionally, water extract of the crushed Chaga is the frequently used formulation (Koyanma et al. 2008) and is believed to inhibit oxidative DNA damage in human lymphocytes (Park et al. 2004b) and induce G0/G1 arrest and apoptosis in human hepatoma HepG2 cells (Youn et al. 2008). Recently, the ethanol extract of Chaga is also found to protect hydrogen peroxide-induced damage of DNA in human lymphocytes (Park et al. 2005a) and to quench free radicals (Cui et al. 2005; Kim et al. 2007a; Lee et al. 2007; Zheng et al. 2009c).
In nature, however, I. obliquus is restricted to very cold habitats (40° N–68° N latitude) and grows very slowly. According to an observation, a Chaga with a diameter of more than 10 cm must undergo at least 10–15 years’ growth (Ham et al. 2009), which suggests that Chaga is not a reliable source for industrial production of these bioactive metabolites (Zheng et al. 2009d). Previous attempts to grow this fungus axenically focused on the accumulation of mycelia biomass (Wang et al. 2006), polysaccharides (Chen and Zhang 2005), and melanins (Babitskaia et al. 2000b) with little attention to the production of other secondary metabolites and their subsequent biological activities. Recently, attempts have been made to grow this fungus in a continuously stirring tank reactor (CSTR) in the presence of hydrogen peroxide (Zheng et al. 2009d) in a shake-flask culture under darkness (Zheng et al. 2009c) or in the presence of a fungal elicitor (Zheng et al. 2009a). CSTR culture stressed by hydrogen peroxide resulted in an increased production of melanins and flavonoid aglycones, but hispidin analogs were only minor components (Zheng et al. 2009c). Moreover, pharmacological activities such as the immuno-stimulating effects only reached about 50% of those from Chaga (Zheng et al. 2008b). Under darkness or in the presence of fungal elicitor, I. obliquus produced more amounts of hispidin analogs, leading to an enhanced capacity for scavenging free radicals. Yet the levels of these phenolic compounds in cultured mycelia were still less than those found in Chaga (Zheng et al. 2009d). Moreover, the accumulation of secondary metabolites other than phenolic compounds has not yet been well documented.
Submerged culture of medicinal fungi is believed to be a promising alternative for the efficient production of mycelia and metabolites and has received increasing attention worldwide. However, in spite of several decades of efforts, the production of secondary metabolites by submerged culture of medicinal fungi including I. obliquus is still encountering many biological, physiological, and engineering limitations. The lack of information on submerged cultures of medicinal fungi in bioreactors is significant when compared to a relatively large body of information about those of streptomycetes and microfungi (Tang et al. 2007).This review focuses on the latest achievements in discovering the chemical diversity of bioactive metabolites produced by I. obliquus grown in its natural habitats and submerged cultures. It highlights the progress during the last couple of years in the findings of new metabolites from Chaga and the submerged culture strategies for diversifying and up-regulating the production of these metabolites, aiming to gain some insights into the solutions to improve and diversify the biosynthesis of these bioactive metabolites in bioreactors.
Biologically active metabolites
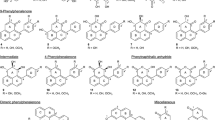
In nature, I. obliquus grows in very cold habitats and is exposed to regular seasonal environmental stresses including freezing temperatures, UV irradiation (Hoshino et al. 1998; Zucconi et al. 2002), and the invasion of various pathogenic microbes (Bolwell et al. 2001). In response to these, I. obliquus has evolved a complex series of integrated defense systems. These include the massive production of antioxidant melanins that form a thick layer outside the woody texture of Chaga, enhanced expression of antioxidant enzymes to create a reduced environment for mycelial growth, and accumulation of a plethora of metabolites that assists in competing successfully the invasion of pathogenic organisms (Shwab and Keller 2008; Zheng et al. 2009e). Chemical investigations have showed that Chaga produces a diverse variety of bioactive metabolites including triterpenoids (1–17), ergosterol (18) and its peroxide (19), sesquiterpene (20) (Taji et al. 2005), benzoic acid derivatives (21–28) (Nakajima et al. 2007), hispidin analogs (29–52) (Lee and Yun 2007) (Fig. 1), melanins (Babitskaia et al. 2000b), and polysaccharides (Mizuno et al. 1999) including β-glucans and heteroglucans (Rhee et al. 2008). The biological activities of these metabolites were summarized in Table 1 and the active fractions or extracts in Table 2.
Antitumoral and immunomodulatory
High incidence of human malicious tumors has always been one of the biggest health threats that is responsible for the annual death of about 7.6 million people worldwide. Studies have shown that a tumor microenvironment influences the functional potential of immune cells by secreting immunosuppressive factors to modify the host immune responses (Chouaib et al. 1997; Chen 1998). These studies raised the possibility that tumors of both mice and human origin can evade immune surveillance by delivering apoptotic death signals to lymphocytes (Fishman et al. 2001). Current strategies for treating malicious tumors commonly involve the combination of surgery and chemotherapy (Efferth and Volm 2005). Yet, surgical removal of breast carcinoma, colon carcinoma, and osteogenic sarcoma, for instance, is frequently followed by metastases to the lung, liver, and other organs of the host (Kimnur et al. 2004). On the other hand, chemotherapy used for the control of tumor growth and metastases is always incurred by cytotoxicity, especially those administered by a combination of more than two drugs (Moreira et al. 2001). In contrast to the current treatment of human malicious tumors, Chaga is an ideal natural drug chemically containing a plethora of metabolites that selectively induce the apoptosis of tumor cells. Evidences in the past decades have shown that Chaga possesses substantial capacities for inhibiting tumor growth and metastasis not only by stimulating host immunity via enhancing the proliferation of lymphocytes (Zheng et al. 2008b) but also by inducing tumor cell apoptosis through up-regulating the expression of Bax genes and down-regulating Bcl-2 genes (Chen et al. 2007a) without incurring any host cell damage. The antitumoral compounds produced in Chaga include triterpenoids inotodiol (2) (Nakata et al. 2007), lanosta-8,23E-diene-3β,22R,25-triol (6), lanosta-7,9(11),23E-triene-3β,22R, 25-triol (8), ergosterol peroxide (19), benzoic acid derivatives (22–28) (Nakajima et al. 2009a), and hispolon (30) and hispidin (31) (Table 1). In addition, the aqueous Chaga extracts and the fractions derived from these extracts containing polysaccharides and polyphenols have also shown to be active in inhibiting tumor growth and metastasis (Burczyk et al. 1996; Rzymowska 1998; Chen et al. 2007a). These underpin water extracts of Chaga that have been used as an effective formulation since the sixteenth century in most Baltic countries. Despite the breakthrough in the bioassay of metabolites in Chaga in the past decades, the antitumoral activity of a large number of phenolic compounds and lanostane-type triterpenoids has not yet been clarified.
Antiviral
Virus is another class of important human pathogens that not only produce the common influenza, chickenpox, and cold sores but also trigger the outbreak of pandemic Ebola, AIDS, avian influenza, severe acute respiratory syndrome, type A H1N1 influenza and hepatitis, etc. (Margolis et al. 2007). In recent years, the growing incidence of avian flu and type A H1N1 influenza poses ongoing threats to human health, killing numerous people each year worldwide. The current therapy of virus-induced diseases includes chemotherapy inhibiting the key enzymes for viral replication in host cells (Li et al. 2009), vaccination to enhance host immunity (Yang et al. 2009), and the delivery of T cells to the infected host (Kumar et al. 2008). Antiretroviral therapy has led to a significant improvement in the prognosis for virus-infected individuals. These involve targeting the envelope protein, reverse transcriptase, integrase, and protease to block virus infection at the stage of entry, reverse transcription, integration, and maturation (Coffin 1995). However, growing clinical evidences have shown that long-term chemotherapy leads to serious multi-drug resistance and toxic side effects in patients (Li et al. 2009). In addition, the increasing number of serious adverse events in children by vaccination have been reported (Rosenberg et al. 2009), and the perception of T cell delivery as invading pathogens by host is still a problem that hinders its application (Nayak and Herzog 2010). As an effective traditional folk remedy, I. obliquus also shows its potential for treating virus-induced diseases. For example, lignin-like polyphenols from water extracts of Chaga showed effective inhibition of protease of HIV-1 (Ichimura et al. 1998). The charcoal-like surface layer of Chaga exhibited 100% inhibition against human and horse type A and B influenza virus, and triterpenoids betulinic acid (20) and mycosterols were thought to be the active constituents (Kahlos et al. 1996). Evidences also indicated that phenolic compounds hispolon (30) and hispidin (31) effectively inhibited the replication of type A and B influenza virus in host cells, and hispidin (31) inactivated HIV-1 integrase at an IC50 value of 2 μM (Singh et al. 2003). Collectively, the metabolites synthesized by I. obliquus demonstrated substantial capacity for treating virus-induced diseases. Yet, the number of active compounds needs further screening and the mechanisms for their antiviral activity remain unknown. Thus, bioprospecting antiviral compounds from Chaga is of great importance for antiviral drug discovery.
Antimutagenic
Mutations in the DNA sequence of a cell’s genome lead primarily to harmful effects to individuals owing to the genetic disorder (Sawyer et al. 2007). Genetic disorder is the origin of severe genetic diseases such as sickle cell anemia, mental retardation, and Down syndrome. These mutagenic diseases occur in high morbidity in most of the developing countries (Verma 2000), resulting in a substantial reduction in the population’s quality of health. The treatment of mutagenic disorders is currently a big challenge and depends on the development of ‘genetic medicine’ therapies using the transfer of DNA and /or RNA to modify gene expression to correct or compensate for an abnormal phenotype. Despite the efficacy of these technologies in treating experimental models of hereditary disorders, their successful application in the clinic still encounters many obstacles (O'Connor and Crystal 2006). Thus, antimutagenic agents have significant application in preventing mutagenic diseases. Metabolites produced by I. obliquus showed remarkable antimutagenic activity. Bioassay-guided fractionation of Chaga methanol extracts led to the isolation of two antimutagenic compounds inotodiol (2) and 3β-hydroxylanosta-8,24-diene-21-al (4). These two compounds inhibit mutations induced by mutagen MNNG by 80.0% and 77.3%, respectively, in Salmonella typhimurium TA100. They also inhibited 4NQO-induced mutagenesis in S. typhimurium TA98 and TA100 by 52.6–62.0% (Ham et al. 2009). The above example suggests that there is a potential in isolating natural compounds produced by I. obliquus in effectively reducing the incidence of mutagenic diseases. However, a thorough evaluation of antimutagenic activity of I. obliquus is necessary to further elucidate the active components and mechanisms of action.
Antioxidant
Oxidative stress stems from the continuous production of H2O2 primarily in the mitochondrion of all aerobic organisms during oxidative phosphorylation for energy production (Turrens 2003). In normal physiological conditions, production of reactive oxygen species (ROS) can be quenched by antioxidant enzymes. However, severe oxidative stress will lead to DNA damage in cells and induce apoptosis (Evans and Cooke 2004). ROS, such as hydroxyl and superoxide anion radicals, demonstrates a wide variety of pathological effects on cellular processes (Marx 1987). Superoxide anion radical is one of the most reactive free radicals easily combined with nitric oxide (NO) produced by the endothelium, macrophages, neutrophils, and brain synaptosomes, leading to the production of the stable peroxynitrite anion (ONOO−) (Beckman et al. 1990), the highly toxic substance responsible for the subsequent cell injuries (Harman 1997). In addition, hydroxyl radical is also a far more reactive free radical that directly interacts with unsaturated fatty acids in the cell membrane, resulting in lipid peroxidation and thereby dysfunctions of cell membranes (Shen and Qian 2004). Long-term severe oxidative stress also increases substantially the incidence of neurodegenerative (Gilgun-Sherki et al. 2001) and cardiovascular diseases (Madamanchi et al. 2005), cataract (Spector 2000), and autoimmune diseases (Shunichi et al. 2003). I. obliquus produces quite an impressive array of metabolites capable of scavenging free radicals including superoxide anion (SOA), 1,1-diphenyl-2-picrylhydrazyl (DPPH), and 2.2′-azinobis-3-ethylbenzothiazoline-6-sulfonate (ABTS). These include lanostane-type triterpenoids 3β-hydroxylanosta-8,24-diene-21-al (4) (Ham et al. 2009), lanosta-24-ene-3β,21-diol (5) (Taji et al. 2005), benzoic acid derivatives (22–28) (Nakajima et al. 2007, 2009b), hispolon (30), hispidin (31), hispidin analogs inonobilins A, B, and C (32–34), phelligridins D–G (36–39) (Lee et al. 2006), interfunginns A–C (42–44) (Lee and Yun 2007), inoscavins A–C (45, 47, 49), methyl inoscavins A–C (46, 48, 50), davallialactone (51), and methyl davallialactone (52) (Lee and Yun 2006). Of these, compounds 42–44 and 51–52 were more effective in quenching SOA with IC50 values of less than 7 μM (Table 1). These metabolites represent antioxidant principles in the extracts of ethanol (Park et al. 2004b; Cui et al. 2005; Hu et al. 2009), ethyl acetate, and n-butanol (Liang et al. 2009). Studies also showed that polysaccharides fractionated from water extracts of Chaga demonstrate remarkable antioxidant activity (Hu et al. 2009). Evidences showed that Chaga polysaccharides inhibit 80% of Fe2+–Cys-induced lipid peroxidation of mitochondria at a minimum concentration of 200 μg/ml (Song et al. 2008). Treatment with Chaga aqueous extract reduced 40% of DNA damage of human lymphocytes induced by H2O2 (100 μg/ml) at a minimum concentration of 100 μg/ml (Park et al. 2004b). The polyphenolic extract of Chaga demonstrated its scavenging for DPPH and SOA for up to 90% (Cui et al. 2005). Melanins, with chemical moieties of electron donors and acceptors, are powerful antioxidants (Babitskaia et al. 2000a; Shcherba et al. 2000) and have received extensive attention in the past decades owing to their effective protection of human skin against UV irradiation (Paramonov et al. 2004) and low-intensity ionizing radiation (Druzhyna et al. 2001). The combination of metabolites produced by I. obliquus might have applications in reducing the incidence of oxidative stress-induced diseases including cataract, hypertension (Kwon et al. 2007), cancer (Orzechowski 2007), neurodegenerative (Heo and Lee 2005) and autoimmune diseases (Galli et al. 2005). Chemical diversity and effective scavenging for free radicals potentialize I. obliquus to be a promising source for screening lead compounds for antioxidant drug discovery.
Anti-inflammatory
Inflammatory responses are triggered by cytokines that are produced primarily by leucocytes. Excessive production of cytokines IL-1β, IL-6, TNF-α, and NO leads to serious cell damage (Lin et al. 2004). Water extract, 80% ethanol, and ethyl acetate extracts of Chaga inhibited the production of these cytokines by murine macrophages (Van et al. 2009). Chaga extracts by ethanol at 80°C showed 81.2% inhibition of platelet aggregation. Systematic isolation and purification resulted in the identification of anti-inflammatory peptide with a molecular mass of 365 Da and a sequence of Trp-Gly-Cys (Hyun et al. 2006). However, other metabolites with anti-inflammatory activity in the Chaga extracts remain unknown.
Anti-complementary
Metabolites from I. obliquus also showed anti-complementary activity. Ergosterol (18) and ergosterol peroxide (19) showed strong anti-complementary activity on classical pathway. Ergosterol \( \left( {{\hbox{I}}{{\hbox{C}}_{{5}0}} = {1}.0 \times {1}{0^{ - {6}}}{\hbox{M}}} \right) \) was more active than ergosterol peroxide \( \left( {{\hbox{I}}{{\hbox{C}}_{{5}0}} = { 5}.0 \times {1}{0^{ - {6}}}{\hbox{M}}} \right) \) (Kim et al. 1997).
Other biological activities
Studies also disclosed other than the above-stated biological activities of the metabolites or fractions from I. obliquus. Chloroform extract of Chaga showed remarkable antiproliferative effect against mouse leukemia P388 cells, and inotodiol (2) is the active principle (Nomura et al. 2008). Hispolon (30) showed cell proliferation inhibitory effect against human epidermoid KB cell at an IC50 value of 4.62 μg/ml (Chen et al. 2006). Trametenolic acid (3) suggested inhibition to the growth of Bacillus subtilis at a minimum concentration of 2.5 μg/ml (Keller et al. 1995). Oral administration by methanol extract of Chaga at doses of 100 and 200 mg/kg substantially reduced the frequencies of acetic acid-induced stretching episodes and the subsequent abnormal constriction in mice (Park et al. 2005b). Oral treatment of β-glucan, heteroglucan, and their protein complexes abated blood glucose level for 3–48 h in type I diabetic mice (Mizuno et al. 1999) and decreased the serum glucose level in streptozotocin-induced diabetic rats (Cha et al. 2005). Oral administration of ethyl acetate extract of Chaga evidently suppressed the blood glucose level in alloxan-induced diabetic mice. Bioassay-guided fractionation resulted in the finding of active principles inotodiol (2) and 3β-hydroxylanosta-8,24-diene-21-al (4) able to inhibit amylase activity and scavenge free radicals (Lu et al. 2009). I. obliquus also demonstrated immunomodulatory effects. Oral administration of Chaga water extract at a dosage of 100 mg/kg prevented body weight reduction induced by cyclophosphamide and stimulated the proliferation of peripheral lymphocytes (Zheng et al. 2008b). It has also been reported that lanosterol analogs showed regulatory effects on the biosynthesis of cholesterol (Frye and Leonard 1999). Chemical diversity of lanostane-type triterpenoids produced by I. obliquus makes it possible to be a source for the discovery of drugs reducing cholesterol-induced human hyperlipidemia.
Production of bioactive metabolites by submerged cultures
The presence of metabolites with chemical and pharmacological diversities in Chaga manifests I. obliquus to be an ideal medicinal fungus for drug discovery. However, the very slow formation of Chaga in natural habitats shows that these pharmacologically important metabolites cannot be produced for therapeutic purposes using naturally growing I. obliquus (Zheng et al. 2009d, e). Thus, culturing I. obliquus is the only solution for large-scale production of the metabolites similar to those from Chaga. Early submerged culture of I. obliquus focused only on the accumulation of mycelial biomass in which suitable growth conditions were probed in carbon/nitrogen ratio, pH, and light intensity by shake-flask platform (Jiang et al. 2004). Later, sucrose and corn powder and pH between 5 and 7 were found to be beneficial for mycelial growth, whereas light irradiation at an intensity of 500 lx reduced the production of mycelial biomass (Chen and Zhang 2005). Recently, growth conditions were further optimized by duality quadratic regress composite intersect design and orthogonal design for the maximum production of mycelial biomass, which resulted in 19.8 and 13.57 g/l dried mycelia in the media containing reduced sugar plus yeast powder and corn juice at a pH value of 6.0 and in those containing glucose and yeast powder at a pH value of 6.0, respectively (Wang et al. 2006).
Targeting the production of melanins
Melanins are the most conspicuous metabolites produced by I. obliquus. Previous attempts for producing melanins in submerged cultures disclosed that the presence of copper ions (0.008%), pyrocatechol (1.0 mM), and tyrosine (20 mM) stimulated melanogenesis (Babitskaia et al. 2000b) and found that melanins produced in submerged cultures of I. obliquus belonged to eumelanins that physiochemically differed from allomelanins in Chaga (Kukulianskaia et al. 2002). Recently, Zheng et al. (2009e) showed that the presence of hydrogen peroxide in submerged cultures of I. obliquus resulted in a substantial enhancement in melanin production, and melanogenesis was inhibited by supplementing arbutin, which suggests a DOPA pathway for melanin synthesis (Zheng et al. 2009d). It is believed that melanins produced by I. obliquus in submerged cultures are antioxidant and genoprotective (Babitskaia et al. 2000a, b), yet further experiments are needed to elucidate the differences between eumelanins and allomelanins in biological activities.
Targeting the production of polysaccharides
Production of polysaccharides was another early target for the submerged culture of I. obliquus. It has been reported that polysaccharides from submerged cultures showed similar hypoglycemic (Mizuno et al. 1999) and antitumoral potential with those obtained from Chaga, and even more effective in antioxidant activity (Song et al. 2008). Kim et al. (2005) showed that intracellular polysaccharides (IPS) from cultured mycelia of I. obliquus suggested a higher potential for stimulating the proliferation of lymphocytes than that of exopolysaccharides (EPS) (Kim et al. 2005). IPS from submerged culture of the fungus showed no direct cytotoxicity against a variety of tumor cell lines in vitro but a remarkable inhibition in vivo on the growth of B16F melanoma-bearing mice (Kim et al. 2007b). Following these findings, culture conditions for the maximum production of EPS were screened. These included the carbon and nitrogen sources and pH value that favored EPS accumulation by which to increase EPS up to 7.8 g/l in the media containing maltose (2%) and peptone (0.4%) at a pH of 5.5 (Chen et al. 2007b) and up to 3.85 g/l in the media containing corn syrup (2.11%), soluble starch (2.23%), yeast powder (0.51%), and peptone (0.41%) with a pH value of 5.0 (Zhang et al. 2009). Recent endeavor was also made to optimize the conditions for the maximum production of IPS. Zhang (2008) showed that UV irradiation (45 W, 30 cm) for 3 min increased IPS accumulation up to 2.39%.The above studies have improved the culture conditions for polysaccharide production. Further experiments are still needed to investigate the chemical structure and pharmacological activities of polysaccharides, and the links between monosaccharides, and to identify whether increased production of polysaccharides correlated with enhanced potential of pharmacological activities.
Targeting the production of triterpenoids and steroids
Attention for metabolite accumulation has also been directed to the production of triterpenoids and steroids. Shin et al. (2001, 2002) isolated lanosterol, ergosterol, and ergosterol peroxide, 3β-22-dihydroxy-lanosta-7,9(11),24-triene, from the mycelia grown in the media containing glucose (2%), malt extract (0.5%), and yeast (0.5%) without modifying the pH after autoclaving. Xiang et al. (2006) determined lanosterol, inotodiol, 3β-hydroxyl-lanosta-8-en-24-methylene, 9(11)-dihydroergosta-benzoate methyl ester, ergosterol, and ξ-ergosterol from the mycelia grown in the media containing glucose (2.5%), peptone (0.3%), and yeast extract (0.1%) without modifying the pH after autoclaving and showed that lanosterol content was considerably less than ergosterol. Recently, I. obliquus from the same medium was also evaluated for the bioconversion from lanosterol to ergosterol, which determined the intermediates of ergosterol biosynthesis including 24-methylene dihydrolanosterol, 4,4-dimethylfecosterol, 4-methylfecosterol, and ergosta-7,22-dien-3-ol in the presence of AgNO3. Following the presence of these intermediates, lanosterol accumulation, which was very low in the absence of AgNO3, was obviously increased together with a reduced activity of 3-hydroxyl-3-methylglutaryl coenzyme A reductase (HMGCoAR) and the subsequent slowing down in sterol metabolism. The above observations suggested that a higher amount of Ag+ inhibits the activity of enzymes responsible for sterol metabolism (Zheng et al. 2007b, 2008a). These findings highlighted the mechanisms differing in chemical profiles between lanosterol analogs biosynthesized in submerged culture and natural habitats. However, mechanisms in up-regulating lanosterol analogs, particularly those with pharmaceutical importance, remain unclear.
Targeting the production of phenolic compounds
Production of phenolic compounds by I. obliquus has recently received much attention due to their prominent antioxidant activities. Zheng et al. (2007a) showed that supplementation of l-tyrosine and water extracts of Aspergillus flavus and Mucor racemosus to the culture of I. obliquus resulted in an increased production of glycosides of quercetin, naringenin, kaempferol, and isorhamnetin together with enhanced capacities for scavenging DPPH, SOA, and hydroxyl radicals. Yang and Zheng (2007) optimized culture medium for producing hydrolyzable tannins and showed that supplementing Cu2+, Co2+, Zn2+, and Mn2+ at a concentration of 0.8, 1.6, 1.6, and 1 mM, respectively, up-regulated the accumulation of hydrolyzable tannins in the medium containing glucose and peptone with a pH value of 5.5. To clarify the difference in phenolic profiles between Chaga and cultured mycelia, phenolic compounds were extracted and compared. It showed that those in Chaga comprised mainly of hispidin analogs concomitant with the presence of minor benzoic acid derivatives, whereas those in cultured mycelia were featured by the dominant presence of glycosylated flavonoids and benzoic acid derivatives; hispidin analogs were determined as the minor components. Further, the capacity for resisting body weight reduction in immuno-suppressed mice by total phenolic compounds from cultured mycelia reached only half of that by total phenolic compounds from Chaga (Zheng et al. 2008b). Recently, the production of phenolic compounds by I. obliquus was conducted in CSTR in the presence of hydrogen peroxide only or in the presence of hydrogen peroxide and arbutin simultaneously, which resulted in the accumulation primarily of flavonoid aglycones and benzoic acid derivatives. Hispidin analogs were still the minor components (Zheng et al. 2009d). Following oxidative stress, I. obliquus enhanced the expression of superoxide dismutases and catalase together with a reduced accumulation of phenolic compounds due to their consumption for free radical scavenging (Zheng et al. 2009e).
Hispidin and flavonoids are biosynthesized via phenylpropanoid pathway, which all start with the precursor phenylalanine and produce the intermediate p-coumaric acid in the presence of phenylalanine ammonia lyase (PAL) (Nambudiri et al. 1973; Dewick 2003; Jiang et al. 2005), and p-coumaric acid can either be transformed to naringenin chalcone (flavonoids) in the presence of chalcone synthase (Moore et al. 2002) or to caffeate in the presence of p-coumaric acid hydroxylase, leading to the biosynthesis of hispidin (Nambudiri et al. 1973). Zhao et al. (2009) showed that p-coumaric acid was predominantly converted into flavonoid aglycones and benzoic acid derivatives under oxidative stress and less transformed to hispidin analogs, which contradicts those growing in natural habitats with phenolic profile featured by the predominant presence of hispidin analogs (Zheng et al. 2008b). In order to search for factors favoring the involvement of p-coumaric acid to the biosynthesis of hispidin analogs, Zheng et al. (2009c) examined the NMR profiles of phenolic compounds from cultured mycelia grown under different light conditions, compared these to those determined in Chaga using NMR-based metabonomic analysis, and found that darkness was beneficial to the biosynthesis of hispidin analogs, particularly davallialactone, methyl davallialactone, and phelligridins, with 1H NMR spectral features close to those of Chaga. In addition, phenolic compounds from cultured mycelia grown in darkness showed a higher potential in scavenging free radicals than those found in day, blue, and red light and concluded that daylight and darkness tend to be beneficial to the accumulation of phenolic compounds. These findings demonstrate that light regulates the biosynthesis of hispidin analogs in I. obliquus, and the light-induced responses are reasonably mediated by white-collar-protein-like photoreceptors capable of initiating signal transduction pathways propitious to the accumulation of hispidin analogs. More recently, Zheng et al. (2009a) conducted further experiments to analyze the effects of fungal elicitor on the production of polyphenols by I. obliquus under darkness and showed that fungal elicitor enhanced the biosynthesis of hispidin analogs and the subsequent capacity of antioxidant activity of total phenolic compounds. Evidences indicated that this enhancement was mediated by an increased production of nitric oxide able to trigger an enhanced expression of PAL, leading to biosynthesis of hispidin analogs. Recently, studies have also been directed to the bioassay of phenolic compounds from submerged cultures of I. obliquus. Sun et al. (2008) showed that the dried material of culture broth, with the constituents mainly being phenolic compounds, demonstrated remarkable antihyperglycemic and antilipidperoxidative effects against alloxan-induced diabetes in mice. The phenolic compounds from submerged culture demonstrated similar or higher potentials in enhancing the tolerance of lead-treated mice to hypoxia than those by Chaga (Zheng et al. 2009b) and the capacities for scavenging free radicals (Zheng et al. 2007a, 2009a, c, d, e; Zhao et al. 2009). These studies uncovered some of the factors affecting the biosynthesis of phenolic compounds by I. obliquus under submerged culture conditions and elucidated the mechanisms leading to the differences in phenolic profiles and biological activities between cultured mycelia and Chaga. Further experiments should be conducted in clarifying the factors affecting the involvement of p-coumaric acid, particularly the signal transduction leading to the gene expression of p-coumaric acid hydroxylase and the enzymes responsible for hispidin biosynthesis. In addition, it is also important to verify the pharmacological activities of the phenolic compounds from submerged culture for evaluating their pharmaceutical significance.
Problems, strategies, and prospects
Current achievements in the study of I. obliquus provide promising prospects not only in the chemical diversity of bioactive metabolites but also in the up-regulation of these metabolites in its submerged cultures. However, as far as the reactors used are concerned, nearly all of the cultures were performed by shake-flasks, where culture parameters such as dissolved oxygen concentration and dynamic changes of pH value can neither be monitored nor controlled (Zheng et al. 2009d). In addition, the configuration of bioreactor greatly affects broth rhenology and thereby oxygen mixing and transfer, leading to the changes of metabolic profiles (Taticek et al. 2004). Thus, the culture conditions probed by shake-flask cultures cannot be applied directly for the scale-up culture of I. obliquus. Further, other factors including shearing force is also involved in the accumulation of mycelial biomass and metabolites, which have not yet been well documented. Thus, it is essential to optimize the growth conditions of the fungus in CSTR for large-scale fermentation by a combined evaluation of mycelial biomass, metabolites and biological activities. In many groups of bacteria, fungi, and plants, there are dozens of secondary metabolite pathways that are not expressed under standard laboratory growth conditions (Pettit 2009), and the presence or absence of environmental factors is critical to the synthesis of secondary metabolites (Knight et al. 2003). Apparently, potentially interesting gene clusters in I. obliquus, while possibly expressing metabolites that increase competitiveness in its natural habitats, can remain silent in its submerged cultures. Based on the current data on submerged culture of the fungus, the differences in growth conditions with natural habitats are the major obstacles for accumulating bioactive metabolites. In this context, strategies aiming at diversification and up-regulation of these metabolites tend either to vary culture conditions to turn on these pathways or to mimic natural microbial environments by growing I. obliquus in the presence of other microbes. Varying culture conditions, i.e., imposing an oxidative stress or programmatically changing growth temperature, does not prove to be cost-effective in that part of metabolites, particularly phenolic compounds will be consumed for resisting the imposed oxidative stresses (Zheng et al. 2009d). In comparison, co-culture with other microbes seems to be a feasible protocol to up-regulate bioactive metabolites by I. obliquus. Zheng et al. showed that simultaneous inoculation of I. obliquus and medicinal fungus Phellinus punctatus at a ratio of 5:1 (w/w) into submerged culture resulted in a reduced production of mycelial biomass (nearly one third reduced) but a substantial increase in the activity of HMGCoAR, PAL, and TAL, the key enzymes responsible for the biosynthesis of triterpenoids, steroids, phenolic compounds, and melanins, respectively, followed by more complex NMR profiles and enhanced production of extracellular melanins , intra- and extracellular phenolic compounds, and triterpenoids (unpublished data). Moreover, culture broth and ethanol/acetone extracts of mycelia from mixed culture all showed significant increase in scavenging free radicals and selective cytotoxicity to HeLa cells (unpublished data). These data evidenced the possibility of diversifying and up-regulating metabolites in submerged culture of the fungus by co-incubation with other microbes. However, the production of adequate quantities of the active compounds needed for drug discovery may require extensive media optimization and scale-up. These include systematic approaches to manipulate the physiology of I. obliquus to activate its natural product machinery by blocking some pathways expressed in submerged cultures and triggering the pathways expressed in nature under laboratory conditions.
To date, at least 20 lanostane-type triterpenoids, 15 flavonoids, 9 benzoic acid derivatives, 10 hispidin analogs, melanins, and polysaccharides were identified or isolated from submerged cultures of I. obliquus. More importantly, metabolites from culture broth and mycelial extracts all showed substantial tumor cytotoxic, hypoglycemic (Cha et al. 2005; Sun et al. 2008), antilipidperoxidative (Sun et al. 2008), immunomodulatory (Zheng et al. 2008b), antioxidant (Zheng et al. 2009c, d, e), and tolerance-enhancing activities (Zheng et al. 2009b). These evidenced that some of the pathways can still be expressed in submerged culture conditions, particularly in the presence of elicitors and oxidative stress. However, most of the desired pathways are not expressed in response to the variation of culture conditions, and the factors inactivating or reducing the expression of these pathways remain unclear.
In higher plants, a conspicuous feature of resistance to the attempted pathogen attack is the synthesis of NO, the signal molecule able to drive the expression of a battery of redox-regulated defense genes (Feechan et al. 2005). S-Nitrosylation, the addition of a NO moiety to a specific cysteine thiol, to form an S-notrosothiol, has emerged as a principal mechanism by which NO orchestrates cellular functions in Arabidopsis thaliana. The associated molecular mechanisms by which NO modulates these diverse cellular responses involve S-nitrosylation of salicylic acid binding protein 3 at cysteine (C) 280. The binding of NO suppresses the immune activator salicylic acid and carbonic anhydrase (CA) activity of this protein, leading to a reduced resistance against pathogen infection. Thus, S-nitrosylation could contribute to a negative feedback loop that governs the plant defense responses (Wang et al. 2008). It has been shown that NO also mediates fungal elicitor-enhanced biosynthesis of hispidin analogs by I. obliquus (Zheng et al. 2009a). Thus, it is highly necessary to investigate whether a similar negative feedback loop exists in I. obliquus that modulates the immune response against the invasion of pathogenic microorganisms and subsequently reduces the production of secondary metabolites.
Synthesis of bioactive metabolites by I. obliquus involves the expression of enzymes in a plethora of pathways, leading to the production of a wide variety of compounds. Rapid and accurate qualification and quantification of these compounds are the preconditions for determining the changes of metabolic profiles of I. obliquus grown in different culture conditions (Zheng et al. 2009c). Metabonomics-based approaches, capable of measuring the dynamic multiparametric responses of living systems to the internal and external stimuli (Nicholson et al. 1999), are claimed to evaluate comprehensively the multiparametric metabolic responses to all stimuli and gene modification (Gao et al. 2008). High-performance liquid chromatography combined with mass spectroscopy (HPLC/MS) provides an authentic platform for identifying metabolites, but establishing suitable conditions and subsequent metabolite isolation and identification are time-consuming and challenging processes. As one of the major techniques in metabonomics, NMR spectroscopy has the disadvantage of low detection limits but with many other advantages over HPLC/MS in that NMR measurements are non-destructive and non-selective and it is feasible to acquire profiles of a comprehensive range of organic metabolites (Li et al. 2007). It is encouraging that this approach has been tentatively used in evaluating the effect of light and fungal elicitor on the biosynthesis of phenolic compounds and well described the metabolites leading to the changes in response to different environmental factors (Zheng et al. 2009a, c). Taken together, it is reasonably believed that biosynthesis of bioactive metabolites by I. obliquus will be diversified towards the desired pathways when we successfully combine gene manipulations and signal transductions with NO-mediated feedback loop of immune response and NMR-based metabonomic analysis.
I. obliquus has already played a pivotal role in treating various human diseases. More thorough exploitation of its metabolic potential via the addition of fungal elicitor or co-culture with other fungi is evidencing to be the cost-effective protocol to enlarge its libraries of bioactive metabolites. With countless fungal elicitors or combinations with other microbes and increasingly sophisticated chemical isolation and structure determination methods, the pharmaceutical potential of I. obliquus for drug discovery seems quite promising.
References
Babitskaia VG, Shcherba VV, Filimonova TV, Grigorchuk EZ (2000a) Melanin pigments of the fungi Paecilomyces variotii and Aspergillus carbonarius. Prikl Biokhim Mikrobiol 36:153–159
Babitskaia VG, Shcherba VV, Ikonnikova NV (2000b) Melanin complex of the fungus Inonotus obliquus. Prikl Biokhim Mikrobiol 36:439–444
Beckman JS, Beckman TW, Chen J, Marshall PA (1990) Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Nat Acad Sci 87:1620–1624
Bolwell PP, Page A, Pislewska M, Wojtaszek P (2001) Pathogenic infection and the oxidative defenses in plant apoplast. Protoplasm 217:20–32
Burczyk J, Gawron A, Slotwinska M, Smietana B, Terminska K (1996) Antimitotic activity of aqueous extracts of Inonotus obliquus. Boll Chim Farm 135:306–309
Cha JY, Jun BS, Lee CH, Yoo KS, Moon C, Cho YS (2005) Hypoglycemic and antioxidant effects of fermented Chaga mushroom (Inonotus obliquus) on streptozotocin-induced diabetic rats. Life Sci Bull 10:809–818
Chen L (1998) Immnological ignorance of silent antigens as an explanation of tumor evasion. Immunol Today 19:27–30
Chen Y, Zhang L (2005) Conditions for mycelial growth of Inonotus obliquus. Edible Fungi Chin 24:8–9
Chen W, He F, Li Y (2006) The apoptosis effect of hispolon from Phellinus linteus (Berkeley and Curtis) Teng on human epidermoid KB cells. J Ethnopharmacol 105:280–285
Chen C, Zheng W, Gao XW, Xiang X, Sun D, Wei J, Chu C (2007a) Aqueous extract of Inonotus obliquus (Fr.) Pilat (Hymenochaetace) significantly inhibits the growth of sarcoma 180 by inducing apoptosis. Am J Pharmcol Toxicol 2:10–17
Chen CF, Xiang XY, Qi G, Zheng WF (2007b) Study on growth medium favoring the accumulation of exopolysaccharides in submerged culture of Inonotus obliquus. Chin Tradit Herbal Drugs 38:358–361
Chouaib S, Asselin-Paturel C, Mai-Couaib F, Caignard A, Baly JY (1997) The host-tumor immune conflict: from immunosuppression to resistance and destruction. Immunol Today 18:493–497
Coffin JM (1995) HIV population dynamics in vivo: implications for genetic variation, pathogenesis, and therapy. Science 267:483–489
Cui Y, Kim DS, Park KC (2005) Antioxidant effect of Inonotus obliquus. J Ethnopharmacol 96:79–85
Dewick PM (2003) Medicinal natural products: a biosynthetic approach. In: Press JWSI (ed), New York, p 130–1332
Druzhyna MO, Burlaka AP, Sidorik EP (2001) Application of fungus melanin for regulation of oxygen radicals generation under continuous exposure to low intensity ionizing radiation. Exp Oncol 23:181–182
Efferth T, Volm M (2005) Pharmacogenetics for individualized cancer chemotherapy. Pharmacol Ther 107:155–176
Evans MD, Cooke MS (2004) Factors contributing to the outcome of oxidative damage to nucleic acids. Bioessays 26:533–542
Feechan A, Kwon E, Yun B-W, Wang Y, Pallas JA, Loake GJ (2005) A central role for S-nitrosothiols in plant disease resistance. Proc Nat Acad Sci 31:8054–8059
Fishman D, Irena B, Kellman-Pressman S, Karas M, Segal S (2001) The role of MHC class I glycoprotein in the regulation of induction of cell death in immunocytes by malignant melanoma cells. Proc Nat Acad Sci 98:1740–1744
Frye LL, Leonard DA (1999) Lanosterol analogues: dual-action inhibitors of cholesterol biosynthesis. Crit Rev Biochem Mol Biol 34:123–140
Galli F, Piroddi M, Annetti C, Aisa C, Floridi E, Floridi A (2005) Oxidative stress and reactive oxygen species. Contrib Nephrol 149:240–260
Gao X, Ge H, Zheng W, Tan R (2008) NMR-based metabonomics for detection of Helicobacter pylori infection in gerbils: which is more descriptive? Helicobacter 13:103–111
Gilgun-Sherki Y, Melamed E, Offen D (2001) Oxidative stress induced-neurodegenerative diseases: the need for antioxidants that penetrate the blood brain barrier. Neurophamacol 40:959–975
Gonindard C, Bergonzi C, Denier C, Sergheraert C, Klaebe A, Chavant L, Hollande E (1997) Synthetic hispidin, a PKC inhibitor, is more cytotoxic towards cancer cells than normal cells in vitro. Cell Biol Toxicol 13:141–153
Ham SS, Kim SH, Moon SY, Chung MJ, Cui CB, Han EK, Chung CK, Choe M (2009) Antimutagenic effects of subfractions of Chaga mushroom (Inonotus obliquus) extract. Mut Res/Genet Toxicol Environ Mutagen 672:55–59
Harman D (1997) Role of free radical reactions in aging and disease. J Geriat Dermatol 5:114–127
Heo HJ, Lee CY (2005) Epicatechin and catechin in cocoa inhibit amyloid beta protein induced apoptosis. J Agr Food Chem 53:1445–1448
Hoshino T, Tronsmo AM, Matsumoto N, Araki T, Georges F, Goda T, Ohgiya S, Ishizaki K (1998) Freezing resistance among isolates of a psychrophilic fungus, Typhula ishikariensis, from Norway. Proc NIPR Symp Polar Biol 11:112–118
Hu H, Zhang Z, Lei Z, Yang Y, Sugiura N (2009) Comparative study of antioxidant activity and antiproliferative effect of hot water and ethanol extracts from the mushroom Inonotus obliquus. J Biosci Bioeng 107:42–48
Huang NL (2002) A mysterious medicinal fungus in Russia: Inonotus obliquus. Edible Fungi Chin 21:7–8
Hyun KW, Jeong SC, Lee DH, Park JS, Lee JS (2006) Isolation and characterization of a novel platelet aggregation inhibitory peptide from the medicinal mushroom, Inonotus obliquus. Peptides 26:1173–1178
Ichimura T, Watanabe O, Maruyama S (1998) Inhibition of HIV-1 protease by water-soluble lignin-like substance from an edible mushroom, Fuscoporia obliqua. Biosci Biotechnol Biochem 62:575–577
Jiang YJ, Lu HZ, Xie BG (2004) Studies on cultivated character of Inonotus obliquus. Fujian J Agr Sci 19:92–95
Jiang H, Wood KV, Morgan JA (2005) Metabolic engineering of the phenylpropanoid pathway in Saccharomyces cerevisiae. Appl Environ Microbiol 71:2962–2969
Kahlos K, Lesnaua A, Lange W, Lindequist U (1996) Preliminary tests of antiviral activity of two Inonotus strains. Fitoterapia 67:344–347
Keller AC, Maillard MP, Hostettmann K (1995) Antimicrobial steroids from the fungus Fomitopsis pinicola. Phytochem 41:1041–1046
Kim DS, Baek N-I, Oh SR, Jung KY, Lee IS, Kim JH, Lee HK (1997) Anticomplementary activity of ergosterol peroxide from Naematoloma fasciculare and reassignment of NMR data. Arch Pharm Res 20:201–205
Kim YO, Han SB, Lee HW, Ahn HJ, Yoon YD, Jung JK, Kim HM, Shin CS (2005) Immuno-stimulating effect of the endo-polysaccharide produced by submerged culture of Inonotus obliquus. Life Sci 77:2438–2456
Kim HG, Yoon DH, Kim CH, Shrestha B, Chang WC, Lim SY, Lee WH, Han SG, Lee JO, Lim MH, Kim GY, Choi S, Song WO, Sung JM, Hwang KC, Kim TW (2007a) Ethanol extract of Inonotus obliquus inhibits lipopolysaccharide-induced inflammation in Raw 264.7 macrophage cells. J Med Food 10:80–89
Kim YO, Park HW, Kim JH, Lee JY, Moon SH, Shin CS (2007b) Anti-cancer effect and structural characterization of endo-polysaccharide from cultivated mycelia of Inonotus obliquus. Life Sci 79:72–80
Kimnur Y, Taniguchi M, Baba K (2004) Antitumor and antimetastatic activities of 4-hydroxyderricin isolated from Angelica keiskei roots. Planta Med 70:211–219
Knight V, Sanglier JJ, Ditullio D, Braccili S, Bonner P, Waters J, Jughes D, Zhang L (2003) Diversitying microbial natural products for drug discovery. Appl Microbiol Biotechnol 62:466–458
Koyanma T, Gu Y, Taka A (2008) Fungal medicine, Fuscoporia obliqua, as a traditional herbal medicine: its bioactivities, in vivo testing and medicinal effects. Asian Biomed 2:459–469
Kukulianskaia TA, Kurchenko NV, Kurchenko KP, Babitskaia VG (2002) Physicochemical properties of melanins produced by Inonotus obliquus (“Chagi”) in the nature and the cultivated fungus. Prikl Biokhim Mikrobiol 38:68–72
Kumar P, Ban HS, Kim SS, Wu H, Pearson T, Greiner DL, Laouar A, Yao J, Haridas V, Habiro K, Yang YG, Jeong JH, Lee KY, Kim YH, Kim SW, Peipp M, Fey GH, Manjunath N, Shultz LD, Lee SK, Shankar P (2008) T cell-specific siRNA delivery suppresses HIV-1 infection in humanized mice. Cell 134:566–568
Kwon YI, Apostolidis E, Shetty K (2007) In vitro studies of eggplant (Solanum melongena) phenolics as inhibitors of key enzymes relevant for type 2 diabetes and hypertension. Biores Technol 99:2981–2988
Lee IK, Yun BS (2006) Hispidin analogs form the mushroom Inonotus xeranticus and their free radical scavenging activity. Bioorg Med Chem Lett 16:2376–2379
Lee IK, Yun BS (2007) Highly oxygenated and unsaturated metabolites providing a diversity of hispidin class antioxidants in the medicinal mushrooms Inonotus and Phellinus. Bioorg Med Chem Lett 15:3309–3314
Lee IK, Seok SJ, Kim WK, Yun BS (2006) Hispidin derivatives from the mushroom Inonotus xeranticus and their antioxidant activity. J Nat Prod 69:299–301
Lee I-K, Kim Y-S, Jang Y-W, Jung J-Y, Yun B-S (2007) New antioxidant polyphenols from the medicinal mushroom Inonotus obliquus. Bioorg Med Chem Lett 17:6678–6681
Lee I-K, G-s S, Jeon NB, Kang HW, Yun BS (2009) Phellinins A1 and A2, new styrylpyrones from the culture broth of Phellinus sp. Kacc93057P: I. fermentation, taxonomy, isolation and biological properties. J Antibiot 62:631–634
Li L, Wang JN, Ren J, Xiang JF, Tang YL, Liu JX, Han D (2007) Metabonomics analysis of the urine of rats with Qi deficiency and blood stasis syndrome based on NMR techniques. Chin Sci Bull 52:3068–3073
Li J, Tan Z, Tang S, Hewlett I, Pang R, He M, He S, Tian B, Chen K, Yang M (2009) Discovery of dual inhibitors targeting both HIV-1 capsid and human cyclophilin A to inhibit the assembly and uncoating of the viral capsid. Bioorg Medl Chem 17:3177–3188
Liang L, Zhang Z, Wang H (2009) Antioxidant activities of extracts and subfractions from Inonotus obliquus. Intl J Food Sci Nutr 60:175–184
Lin HI, Chu SJ, Wang D, Feng NH (2004) Pharmacological modulation of TNF production in macrophages. J Microbiol Immunol Infect 37:8–15
Lu X, Chen H, Dong P, Fu L, Zhang X (2009) Phytochemical characteristics and hypoglycaemic activity of fraction from mushroom Inonotus obliquus. J Sci Food Agr 90:276–280
Madamanchi NR, Vendryov A, Runge MS (2005) Oxidative stress and vascular disease. Arterioscl Thromb Vas Biol 25:29–38
Margolis TP, Eifman FL, Leib D, Pakpour N, Apakupakul K, Imai Y, Voytek C (2007) Spontaneous reactivation of herpes simplex virus type 1 in latently infected murine sensory ganglia. J Virol 81:11069–11074
Marx JL (1987) Oxygen free radicals linked to many diseases. Science 35:519–526
Mizuno T, Zhuang C, Abe K, Okamoto H, Kiho T, Ukai S, Leclerc S, Meijer L (1999) Antitumor and hypoglycemic activities of polysaccharides from the sclerotia and mycelia of Inonotus obliquus (Pers.: Fr) Pil. (Aphyllophoromycetideae). Intl J Med Mush 1:301–316
Moore BS, Hertweck C, Hopke JN, Izumikawa M, Kalaitzis JA, Nilsen G, O'Hare T, Piel J, Pr S, Xiang L, Austin MB, Noel JP (2002) Plant-like biosynthetic pathways in bacteria: from benzoic acid to chalcone. J Nat Prod 65:1956–1962
Moreira A, Lobato R, Morais J, Silva S, Ribeiro J, Figueira A, Vale D, Sousa C, Araujo F, Fernandes A, Olivera J, Passos-Coelho J (2001) Influence of the interval between the administration of doxorubicin and paclitaxel on the pharmaco-kinetics of these drugs in patients with locally advanced breast cancer. Cancer Chemother Phamacol 48:333–337
Nakajima Y, Sato Y, Konishi T (2007) Antioxidant small phenolic ingredients in Inonotus obliquus (persoon) Pilat (Chaga). Chem Pharm Bull 55:1222–1226
Nakajima Y, Nishida H, Matsugo S, Konishi T (2009a) Cancer cell cytotoxicity of extracts and small phenolic compounds from Chaga [Inonotus obliquus (Persoon) Pilat]. J Med Food 12:501–507
Nakajima Y, Nishida H, Nakamura Y, Konishi T (2009b) Prevention of hydrogen peroxide-induced oxidative stress in PC12 cells by 3,4-dihydroxybenzalacetone from Chaga (Inonotus obliquus (Persoon) Pilat). Free Rad Biol Med 47:1154–1161
Nakata T, Yamada T, Taji S, Ohishi H, S-i W, Tokuda H, Sauma K, Tanaka R (2007) Structure determinaiton of inonotsuoxides A and B and in vivo anti-tumor promoting activity of inotodiol from the sclerotia of Inonotus obliquus. Bioorg Med Chem 15:257–264
Nambudiri AMD, Vance CP, Towers GHN (1973) Effect of light on enzymes of phenylpropanoids metabolism and hispidin biosynthesis in Polyporus hispidus. Biochem J 134:891–897
Nayak S, Herzog RW (2010) Progress and prospects: immune responses to viral vectors. Gene Ther 17:295–304
Nicholson JK, Cnnelly J, Holmes E (1999) “Metabonomics”: understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica 29:1181–1189
Nomura M, Takahashi T, Uesugi A, Tanaka R, Kobayashi S (2008) Inotodiol, a lanostane triterpenoid, from Inonotus obliquus inhibits cell proliferation through caspase-3-dependent apoptosis. Anticancer Res 28:5A 2691–2696
O'Connor TP, Crystal RG (2006) Genetic medicines: treatment strategies for hereditary disorders. Nat Rev Genet 7:261–276
Orzechowski A (2007) Possible implications of redox-sensitive tumour cell transformation; lessons from cell culture studies. Pol J Vet Sci 10:123–126
Paramonov BA, Turkovskii II, Potokin IL, Chebotarev VY (2004) Photoprotective activity of melanin preparations from black yeast-like fungus during UV irradiation of human skin: dependence on the concentration. Bull Exp Biol Med 133:1573–1581
Park I, Chung S, Lee K, Yoo Y, Kim S, Kim G, Song K (2004a) An antioxidant hispidin from the mycleial cultures of Phellinus linteus. Arch Pharm Res 27:615–618
Park YK, Lee HB, Jeon EJ, Jung HS, Kang MH (2004b) Chaga mushroom extract inhibits oxidative DNA damage in human lymphocytes as assessed by comet assay. Biofactors 21:109–112
Park E, Jeon K-I, Byun B-H (2005a) Ethanol extract of Inonotus obliquus shows antigenotoxic effect on hydrogen peroxide induced DNA damage in human lymphocytes. Cancer Prev Res 10:54–59
Park YM, Won JH, Kim YH, Choi JW, Park HJ, Lee KT (2005b) In vivo and in vitro anti-inflammatory and anti-nociceptive effects of the methanol extract of Inonotus obliquus. J Ethnopharmacol 101:120–128
Pettit RK (2009) Mixed fermentation for natural product drug discovery. Appl Microbiol Biotechnol 83:19–25
Rhee SJ, Cho SY, Kim KM, Cha D-S, Park H-J (2008) A comparative study of analytical methods for alkali-soluble β-glucan in medicinal mushroom, Chaga (Inonotus obliquus). LWT-Food Sci Technol 41:545–549
Rosenberg M, Sparks R, McMahon A, Iskander J, Campbell JD, Edwards KM (2009) Serious adverse events rarely reported after trivalent inactivated influenza vaccine (TIV) in children 6–23 months of age. Vaccine 27:4278–4283
Ryvarden L, Gilbertson RL (1993) European polypores. Part 1, 2. Lubrecht & Cramer Ltd, Oslo
Rzymowska J (1998) The effect of aqueous extracts from Inonotus obliquus on the mitotic index and enzyme activities. Boll Chim Farm 137:13–15
Sawyer SA, Parsch J, Zhang Z, Hartl DL (2007) Prevalence of positive selection among nearly neutral amino acid replacements in Drosophila. Proc Natl Acad Sci 104:6504–6510
Shcherba VV, Babitskaia VG, Kurchenko VP, Ikonnikova NV, Kukukianskaia TA (2000) Antioxidant features of fungal melanin pigments. Prikl Biokhim Mikrobiol 36:569–574
Shen XC, Qian ZY (2004) Protection of crocetin on primary culture cardiac myocyte injured by hydroxyl free radicals. Chin Tradit Herbal Drugs 35:657–660
Shin Y, Tamai Y, Minoru T (2001) Chemical constituents of Inonotus obliquus. IV. Triterpene and steroids from cultured mycelia. Eurasian J Forest Res 2:27–30
Shin Y, Tamai Y, Terazawa M (2002) Triterpenoids, steroids, and a new sesquiterpene from Inonotus obliquus (Pers.: Fr.) Pilat. Intl J Med Mush 4:77–84
Shunichi K, Yumiko N, Jun S (2003) Oxidative stress and autoimmnue diseases. J JPN Soc Intl Med 92:1096–1103
Shwab EK, Keller NP (2008) Regulation of secondary metabolite production in filamentous ascomycetes. Mycol Res 112:225–230
Singh SB, Jayasuriya H, Dewy R, Jd P, Dombrowski AW, Zink DL, Guan Z, Collado J, Platas G, Pelaez F, Felock PJ, Hazuda DJ (2003) Isolation, structure, and HIV-1-integrase inhibitory activity of structurally diverse fungal metabolites. J Ind Microbiol Biotechnol 30:721–731
Song Y, Hui J, Kou W, Xin R, Jia F, Wang N, Hu F, Zhang H, Liu H (2008) Identification of Inonotus obliquus and analysis of antioxidation and antitumor activities of polysaccharides. Curr Microbiol 57:454–462
Spector A (2000) Oxidative stress and disease. J Ocul Pharmacol Ther 16:193–203
Sun JE, Ao ZH, Lu ZM, Xu HY, Zhang XM, Dou WF, Xu ZH (2008) Anthihyperglycemic and antilipidperoxidative effects of dry matter of culture broth of Inonotus obliquus in submerged culture on normal and alloxan-diabetes mice. J Ethnopharmacol 118:7–13
Taji S, Yamada T, S-i W, Tokuda H, Sakuma K, Tanaka R (2005) Lanostane-type triterpenoids from the sclerotia of Inonotus obliquus possessing anti-tumor promoting activity. Eur J Med Chem 43:2373–2379
Tang Y, Zhu L, Li H, Li D (2007) Submerged culture of mushrooms in bioreactors—challenges, current state-of-the-art, and future prospects. Food Technol Biotechnol 45:221–229
Taticek RA, Moo-Young M, Legge RL (2004) Effect of bioreactor configuration on substrate uptake by cell suspension cultures of the plant Eschscholtzia californica. Appl Microbiol Biotechnol 33:280–286
Turrens JF (2003) Mitochondrial formation of reactive oxygen species. J Physiol 552:335–344
Van Q, Nayak BN, Reimer M, Jones PJH, Fulcher RG, Rempel CB (2009) Anti-inflammaroty effect of Inonotus obliquus, Polygala senega L., and Viburnum trilobum in a cell sereening assay. J Ethnopharmacol 125:487–493
Verma IC (2000) Burden of genetic disorders in India. Indian J Pediatr 67:893–898
Wang ZH, Huo YF, Wang B, Shen JW (2006) Study on submerged cultures of Inonotus obliquus. Mycosystema 25:461–467
Wang YQ, Feechan A, Yun BW, Shafiei R, Hofmann A, Taylor P, Xue P, Yang FQ, Xie ZS, Pallas JA, Chu CC, Loake GJ (2008) S-Nitrosylation of AtSABP3 antagonizes the expression of plant immunity. J Biol Chem 284:2131–3137
Xiang XY, Zheng WF, Zhang XP (2006) Non-polar constituents in the fermentation products of Inonotus obliquus. Chin Tradit Herbal Drugs 37:670–672
Yang SZ, Zheng WF (2007) Factors affecting the accumulation of hydrolysable tannins in cultured mycelia of Inonotus obliquus. Chin Tradit Herbal Drugs 38:798–803
Yang T, Wang HN, Wang X, Tang JN, Lu D, Zhang YF, Guo ZC, Li YL, Gao R, Kang RM (2009) The protective immune response against infectious bronchitis virus induced by multi-epitope based peptide vaccines. Biosci Biotechnol Biochem 73:1500–1504
Youn M-J, Kim J-K, Park S-Y, Kim Y, Kim S-J, Lee JS, Chai KY, Kim H-J, Cui M-X, So HS, K-y K, Park R (2008) Chaga mushroom (Inonotus obliquus) induces G0/G1 arrest and apoptosis in human hepatoma HepG2 cells. World J Gastroenterol 28:511–517
Youn MJ, Kim JK, Park SY, Kim Y, Park C, Kim ES, Park KI, So HS, Park R (2009) Potential anticancer properties of the water extract of Inonotus obliquus by induction of apoptosis in melanoma B16-F10 cells. J Ethnopharmacol 121:221–218
Zhang LQ (2008) Effect of UV on the growth of Inonotus obliquus and the content of polysaccharides. Jingsen Stud (4):16–19
Zhang ZS, Shi JN, Zhang J, Zhang JP (2009) Optimization of the polysaccharide accumulation by Inonotus obliquus in submerged fermentation. Modern Food Sci Technol 25:298–301
Zhao Y, Miao K, Sun W, Zhang M, Wei Z, Zheng W (2009) Effects of jasmonic acid and hydrogen peroxide on production of phenolic compounds in a melanin-deficient mutant of Phaeoporus obliquus. Mycosystema 28:129–137
Zheng W, Gu Q, Chen C (2007a) Aminophenols and mold-water-extracts affect the accumulation of flavonoids and their antioxidant activity in cultured mycelia of Inonotus obliquus. Mycosystema 26:414–426
Zheng W, Liu T, Xiao X, Qi G (2007b) Sterol composition in field-grown and cultured mycelia of Inonotus obliquus. Acta Pharm Sin 42:750–756
Zheng W, Xiang X, Chen C, Wang Y, Zhao Y, Jiang J, Chu C (2008a) Effects of culture media and three metal ions on the accumulation of lanosterol and ergosterol in cultured mycelia of Inonotus obliquus. Mycosystema 27:438–452
Zheng W, Zhao Y, Zhang M, Yin Z, Chen C, Wei Z (2008b) Phenolic compounds from Inonotus obliquus and their immune stimulating effects. Mycosystema 27:39–47
Zheng W, Miao K, Zhao Y, Zhang M (2009a) Nitric oxide mediates fungal elicitor-enhanced biosynthesis of antioxidant polyphenols in Inonotus obliquus in submerged cultures. Microbiol 155:3340–3348
Zheng W, Yin Z, Chen C, Zhang M, Zhao Y (2009b) Phenolic compounds from submerged cultures of Phaeoporus obliquus enhance tolerance of lead-treated mice. Mycosystema 28:112–119
Zheng W, Zhang M, Zhao Y, Miao K, Jiang H (2009c) NMR-based metabonomic analysis on effect of light on production of antioxidant phenolic compounds in submerged cultures of Inonotus obliquus. Biores Technol 100:4481–4487
Zheng W, Zhang M, Zhao Y, Wang Y (2009d) Accumulation of antioxidant phenolic constituents in submerged cultures of Inonotus obliquus. Biores Technol 100:1327–1335
Zheng W, Zhao Y, Zhang M, Wei Z, Miao K, Sun W (2009e) Oxidative stress response of Inonotus obliquus induced by hydrogen peroxide. Med Mycol 47:814–823
Zucconi L, Ripa C, Selbmann L, Onofri S (2002) Effects of UV on the spores of the fungal species Arthrobotrys oligospora and A. ferox. Polar Biol 25:500–505
Acknowledgements
Financial support was provided by grants Natural Science Foundation of China (30910103907) and Natural Science foundations of Jiangsu Province, China (BK2009084, SBK201040012).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zheng, W., Miao, K., Liu, Y. et al. Chemical diversity of biologically active metabolites in the sclerotia of Inonotus obliquus and submerged culture strategies for up-regulating their production. Appl Microbiol Biotechnol 87, 1237–1254 (2010). https://doi.org/10.1007/s00253-010-2682-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-010-2682-4