Abstract
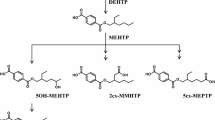
In this study we investigated human metabolism and excretion of DEHP after intravenous exposure. For this purpose we determined the five major DEHP metabolites in urine samples of a volunteer before and after a platelet donation (dual-needle technique). Plateletpheresis procedures are known to cause a significant DEHP exposure. We observed a sharp increase in urinary DEHP metabolite concentrations after the procedure. Maximum concentrations of 5OH-MEHP, 5oxo-MEHP, 5cx-MEPP and MEHP observed 4 h after the procedure were 822, 729, 577 and 388 μg/l respectively. 2cx-MMHP was excreted at highest concentrations after 8 h (201 μg/l). Due to longer elimination half-times, 5cx-MEPP and 2cx-MMHP were the major metabolites excreted in urine 24 h after the exposure. The 24-h-cumulative excretion of 363 μg 5cx-MEPP, 353 μg 5OH-MEHP, 309 μg 5oxo-MEHP, 178 μg MEHP and 133 μg 2cx-MMHP indicates an absolute exposure of our volunteer of about 2.6 mg DEHP. Related to the body weight this equals a dose of 31.6 μg/kg body weight/day. This indicates that current risk or preventive limit values for DEHP such as the RfD of the US EPA (20 μg/kg/day) and the TDI of the European Union (20–48 μg/kg/day) can be exceeded on the day of the plateletpheresis. The amount of the dose excreted in urine, distribution of the metabolites in urine and all other elimination characteristics after intravenous DEHP exposure are comparable to oral exposure. There are no indications that toxicokinetic behaviour and the toxicity of DEHP are fundamentally different after the two routes of exposure. Therefore, toxicological endpoints observed for DEHP after oral application should also be considered relevant for medical procedures causing intravenous DEHP exposure, like apheresis procedures. Especially women in their reproductive age need to be protected from DEHP exposures exceeding the above mentioned preventive limit values.


Similar content being viewed by others
References
AdvaMed (2001) 21-Day repeat dose male reproductive tract study of di(2-ethylhexyl)phthalate (DEHP) administered either intravenously or orally to rats starting at neonatal age 3–5 days, with satellite recovery group through 90 days of age. Study number 11947
Albro PW, Corbett JT, Schroeder JL, Jordan ST, Matthews HB (1982) Pharmacokinetics, interactions with macromolecules and species differences in metabolism of DEHP. Environ Health Perspect 45:19–25
Barr DB, Silva MJ, Kato K, Reidy JA, Malek NA, Hurtz D, Sadowski M, Needham LL, Calafat AM (2003) Assessing human exposure to phthalates using monoesters and their oxidized metabolites as biomarkers. Environ Health Perspect 111(9):1148–1151
Becker K, Seiwert M, Angerer J, Heger W, Koch HM, Nagorka R, Rosskamp E, Schluter C, Seifert B, Ullrich D (2004) DEHP metabolites in urine of children and DEHP in house dust. Int J Hyg Environ Health 207(5):409–417
Buchta C, Bittner C, Hocker P, Macher M, Schmid R, Seger C, Dettke M (2003) Donor exposure to the plasticizer di(2-ethylhexyl)phthalate during plateletpheresis. Transfusion 43(8):1115–1120
Council of Europe (2003) Guide to the preparation, use and quality assurance of blood components. Recommendation No. R (95) 15. Strasbourg, France: Council of Europe Publishing
CSTEE—Scientific committee on toxicity, ecotoxicity and the environment (1998) Opinion on Phthalate migration from soft PVC toys and child-care articles—Data made available since the 16th of June 1998, opinion expressed at the 6th CSTEE plenary meeting, Brussels, 26/27 November 1998
CSTEE—Scientific Committee for Toxicity, Ecotoxicity and the Environment (2004) Opinion on the results of a second Risk Assessment of: Bis(2-ethylhexyl)phthalate [DEHP] Human Health Part. Adopted by the CSTEE during the 41th plenary meeting of 8 January 2004
DFG, Deutsche Forschungsgemeinschaft (2002) Di(2-ethylhexyl)phthalat (DEHP). In: H Greim (Hrsg) Gesundheitsschäd-liche Arbeitsstoffe—Toxikologisch-arbeitsmedizinische Begründungen von MAK-Werten. 35. Ergänzungslieferung, Wiley-VCH
DG SANCO (2002) European Commission, Health & Consumer Protection Directorate-General. Opinion on Medical Devices Containing DEHP Plasticised PVC; Neonates and Other Groups Possibly at Risk from DEHP Toxicity. Adopted by The Scientific Committee on Medicinal Products and Medical Devices on 26 September 2002
Dirven HA, van den Broek PH, Jongeneelen FJ (1993) Determination of four metabolites of the plasticizer di(2-ethylhexyl)phthalate in human urine samples. Int Arch Occup Environ Health 64(8):555–560
EPA—U.S. Environmental Protection Agency (1999) Integrated Risk Information System (IRIS) on Di(2-ethylhexyl)phthalate. National Center for Environmental Assessment, Office of Research and Development, Washington, DC., USA
FDA—U.S. Food and Drug Administration (2001) Safety assessment of di(2-ethylhexyl)phthalate (DEHP) released from medical devices. Center for Devices and Radiological Health, U.S. Food and Drug Administration, 12709 Twinbrook Parkway. Rockville, MD 20852, USA
Federal Medical Council and Paul-Ehrlich-Institute (2001) Guidelines for the collection of blood and blood components and for the use of blood components (Hemotherapy). Deutscher Aerzte Verlag. Cologne, Germany
Fisher JS (2004) Environmental anti-androgens and male reproductive health: focus on phthalates and testicular dysgenesis syndrome. Reproduction 127(3):305–315
Gilsing H-D, Angerer J, Prescher D (2002) Monophthalates with 0xidized C5-carbon in the ester chain: a simple synthetic access to two major metabolites of bis-(2-ethylhexyl)-phthalate. Monatsh Chem 133:1147–1155
Gilsing HD, Angerer J, Prescher D (2003) (2-Ethyl-5-oxo-hexyl) methylthiomethyl phthalate as by-product of the Swern oxidation: improved synthesis of ring-deuterated major metabolites of bis(2-ethylhexyl) phthalate. Monatsh Chem 134(9):1207–1213
Gilsing HD, Angerer J, Prescher D (2005) Convenient and high-yielding preparation procedures for mono(5-carboxy-2-ethylpentyl) phthalate and its ring-deuterated isomer—the “third” major metabolite of bis(2-ethylhexyl)phthalate. Monatsh Chem 136(5):795–801
Health Canada (2002) DEHP in Medical Devices: An Exposure and Toxicity Assessment. Medical Devices Bureau Therapeutic Products Directorate, Health Products & Foods Branch, Health Canada. Revised: February 2002. Irwin Hinberg, Ph.D, Head, Criteria & Assessment, Research & Surveillance Division, Medical Devices Bureau, Therapeutic Products Directorate
Hoppin JA (2003) Male reproductive effects of phthalates: an emerging picture. Epidemiology 14(3):259–260
Kato K, Silva MJ, Reidy JA, Hurtz D III, Malek NA, Needham LL, Nakazawa H, Barr DB, Calafat AM (2004) Mono(2-ethyl-5-hydroxyhexyl) phthalate and mono-(2-ethyl-5-oxohexyl) phthalate as biomarkers for human exposure assessment to di-(2-ethylhexyl) phthalate. Environ Health Perspect 112(3):327–330
Kavlock R, Boeckelheide K, Chapin R, Cunningham M, Faustman E, Foster P et al. (2002) NTP Center for the Evaluation of Risks to Human Reproduktion: phthalates expert panel report on the reproductive and developmantel toxicity of di(2-ethylhexyl)phthalate. Reprod Toxicol 16:529–653
Koch HM, Gonzalez-Reche LM, Angerer J (2003a) On-line cleanup by multidimensional LC-ESI-MS/MS for high throughput quantification of primary and secondary phthalate metabolites in human urine. J Chromatogr B 784:169–182
Koch HM, Rossbach B, Dexler H, Angerer J (2003b) Internal exposure of the general population to DEHP and other phthalates—determination of secondary and primary phthalate monoester metabolites in urine. Environ Res 93(2):177–185
Koch HM, Drexler H, Angerer J (2004a) Internal exposure of nursery-school children and their parents and teachers to di(2-ethylhexyl)phthalate (DEHP). Int J Hyg Environ Health 207(1):15–22
Koch HM, Bolt HM, Angerer J (2004b) Di(2-ethylhexyl)phthalate (DEHP) metabolites in human urine and serum after a single oral dose of deuterium labelled DEHP. Arch Toxicol 78:123–130
Koch HM, Bolt HM, Preuss R, Angerer J (2005) New metabolites of di(2-ethylhexyl)phthalate (DEHP) in human urine and serum after single oral doses of deuterium-labelled DEHP. Arch Toxicol [Epub ahead of print 8Feb 2005]. DOI 10.1007/s00204-004-0642-4
Koch HM, Eckstein R, Weisbach V, Drexler H, Angerer J (in press) Di(2-ethylhexyl)phthalate (DEHP) exposure of voluntary plasma and platelet donors. Int J Hyg Environ Health
Peck CC, Albro PW (1982) Toxic potential of the plasticizer Di(2-ethylhexyl) phthalate in the context of its disposition and metabolism in primates and man. Environ Health Perspect 45:11–17
Preuss R, Koch HM, Angerer J (2005) Biological monitoring of the five major metabolites of di-(2-ethylhexyl)phthalate (DEHP) in human urine using column-switching liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 816(1–2):269–280
Regnier J, Bowden C, Lhuguenot J (2004) Effexts on rat embryonic development in vitro of di-(2-ethylhexyl) phthalate (DEHP) and its metabolites. The Toxicologist CD—An official Journal of the Society of Toxicology 78(1-S):187
Schmid P, Schlatter C (1985) Excretion and metabolism of di(2-ethylhexyl)phthalate in man. Xenobiotica 15(3):251–256
Sharpe RM, Irvine DS (2004) How strong is the evidence of a link between environmental chemicals and adverse effects on human reproductive health?. BMJ 328:447–451
Stroheker T, Cabaton N, Nourdin G, Regnier JF, Lhuguenot JC, Chagnon MC (2005) Evaluation of anti-androgenic activity of di-(2-ethylhexyl)phthalate. Toxicology 208(1):115–121
Wolfe GW, Layton KA (2003) Multigeneration reproduction toxicity study in rats (unaudited draft): Diethylhexylphthalate: Multigenerational reproductive assessment by continuous breeding when administered to Sprague-Dawley rats in the diet. TherImmune Research Corporation (Gaithersburg, Maryland), TRC Study No 7244–200
Acknowledgements
We especially thank the Deutsche Forschungsgemeinschaft (German Research Foundation) and the Bayerische Forschungsstiftung (Bavarian Research Foundation) for their financial support of the project (DFG No.: AN 107/16-4; BF No.: AZ: 565/03).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Koch, H.M., Bolt, H.M., Preuss, R. et al. Intravenous exposure to di(2-ethylhexyl)phthalate (DEHP): metabolites of DEHP in urine after a voluntary platelet donation. Arch Toxicol 79, 689–693 (2005). https://doi.org/10.1007/s00204-005-0004-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-005-0004-x
Keywords
- Metabolism
- Human
- Intravenous
- Exposure
- Apheresis
- Plateletpheresis
- Di(2-ethylhexyl)phthalate (DEHP)
- Mono(2-ethyl-5-hydroxyhexyl)phthalate (5OH-MEHP)
- Mono(2-ethyl-5-oxo-hexyl)phthalate (5oxo-MEHP)
- Mono(2-ethyl-5-carboxypropyl)phthalate (5cx-MEPP)
- Mono[2-(carboxymethyl)hexyl]phthalate (2cx-MMHP)
- Mono(2-ethylhexyl)phthalate (MEHP)