Abstract
Aims/hypothesis
We have previously described a strong correlation between pyruvate cycling and insulin secretion. We have also demonstrated a particularly important role for a pyruvate–isocitrate cycling pathway involving the mitochondrial citrate/isocitrate carrier (CIC) and cytosolic NADP-dependent isocitrate dehydrogenase. CIC requires cytosolic malate as a counter-substrate during citrate and isocitrate export. Thus, considering that the mitochondrial dicarboxylate carrier (DIC) provides an important source of cytosolic malate, we investigated the potential role of DIC in control of glucose-stimulated insulin secretion (GSIS).
Methods
We used pharmacological and small interfering RNA (siRNA) tools to assess the role of DIC in insulin release in clonal insulin-secreting 832/13 cells and isolated rat islets.
Results
Butylmalonate, an inhibitor of malate transport, reduced cytosolic malate and citrate levels, and inhibited GSIS in a dose-dependent manner in 832/13 cells. Suppression of DIC expression resulted in inhibition of GSIS by 5% to 69%, the extent of inhibition of insulin secretion being proportional to the level of Dic (also known as Slc25a10) gene knockdown. The most effective siRNA duplex against Dic did not affect glucose utilisation, glucose oxidation or ATP/ADP ratio, but did suppress glucose-induced increments of the NADPH/NADP+ ratio. Confirmation of our results in primary cultures of isolated rat islets showed that butylmalonate and an adenovirus expressing an siRNA against Dic-inhibited GSIS.
Conclusions/interpretation
Malate transport by DIC may play an important role in GSIS, possibly by providing cytosolic malate as a counter-substrate for citrate and/or isocitrate export by CIC. These studies also suggest that malate transport by DIC is (1) a critical component of NADPH production mediated by pyruvate-cycling and (2) regulates GSIS.
Similar content being viewed by others
Introduction
Glucose-stimulated insulin secretion (GSIS) is biphasic and involves KATP channel-dependent and KATP channel-independent pathways. The KATP channel-dependent pathway or consensus model of GSIS holds that an increase in glucose metabolism causes a rise of the cellular ATP/ADP ratio, which promotes closure of KATP channels, membrane depolarisation and the subsequent activation of L-type voltage-dependent Ca2+ channels [1]. Opening of these channels allows the entry of extracellular Ca2+ and the concomitant rise in cytosolic Ca2+ concentrations triggers insulin release [2]. This KATP channel-dependent pathway is particularly important during the first phase of insulin release, whereas KATP channel-independent mechanisms appear to play a significant role in the second and more sustained phase of insulin release.
Experimental evidence for the KATP channel-independent pathway of GSIS comes from studies showing that glucose can still augment insulin secretion under conditions where the KATP channels are held open by application of diazoxide and where cytosolic Ca2+ was clamped at high levels with high K+ [3]. Similar observations have been made in beta cells, which lack functional KATP channels [4, 5]. Our group, as well as others, has shown an apparent disconnect between nutrient-stimulated increase in the ATP/ADP ratio and insulin release [3–21]. Although ATP is a critical factor in the control of GSIS, an important question arises from these studies: what are the other important metabolic signalling molecule(s) for insulin secretion?
The production of candidate coupling factors such as malonyl-CoA, long-chain-acyl-CoA and NADPH has been shown to depend on the export of mitochondrial metabolites via one of the following mitochondrial carrier proteins: (1) the dicarboxylate carrier (DIC), which translocates malate in exchange for a phosphate; (2) the 2-oxoglutarate carrier, which transports 2-oxoglutarate (also known as α-ketoglutarate) in exchange for malate; and (3) the citrate/isocitrate carrier (CIC), which catalyses an electroneutral exchange of one of three tricarboxylic acids (citrate, isocitrate, cis-aconitate) plus a proton, for another tricarboxylate-H+, a dicarboxylate (malate or succinate) or phosphoenolpyruvate [17, 22]. These mitochondrial carriers play a critical role in intermediary metabolism as they not only provide the necessary source of carbon for fatty acid and cholesterol synthesis, but also facilitate production of sufficient amounts of NADPH, which is required to support these metabolic pathways.
Another important insulin secretion pathway that depends on metabolite transport is pyruvate cycling [12, 15, 17, 19, 23–25]. Pyruvate cycling has been suggested to generate two potentially important signalling molecules for insulin release, α-ketoglutarate and NADPH. NADPH can be produced via one of three pyruvate cycling pathways, the pyruvate/malate pathway, the pyruvate/citrate pathway, or the pyruvate/isocitrate pathway. A key NADPH-producing enzyme for both the pyruvate malate and pyruvate citrate pathways is the cytosolic NADP+-dependent isoform of malic enzyme [12, 26, 27]. The key enzyme for the pyruvate isocitrate pathway is the cytosolic NADP+-dependent isocitrate dehydrogenase (ICDc) [23]. Although strong evidence for a role of the pyruvate–isocitrate pathways exists [12, 17, 19, 23, 25–27], others have shown a role for the other two other pyruvate cycling pathways [28–30]. At present it is unknown which pyruvate cycling pathway(s) is (are) critical for insulin release.
Understanding the mechanism of abnormal insulin secretion is essential to developing novel therapies for type 2 diabetes. However, before we can even begin to develop therapies to improve insulin release in the diabetic state, we first must understand how the beta cell senses glucose under normal physiological conditions. Our group has provided evidence for a novel pathway involved in GSIS. We have shown that suppression of ICDc activity strongly impairs GSIS, pyruvate cycling and NAPDH production [23]. We have also shown that CIC plays a particularly important role in regulation of insulin secretion possibly by providing isocitrate for use by ICDc [17]. DIC would provide malate as an important counter-ion for citrate transport by CIC. In the current study we show that inhibition of DIC with a DIC inhibitor, butylmalonate, or by small interfering RNA (siRNA)-mediated suppression of its gene expression, results in potent inhibition of NADPH production and GSIS. We also show that the inhibitory effects of butylmalonate on GSIS were reversed in isolated rat islets by adding dimethyl malate.
Methods
Cell lines
We used the cell line 832/13 [31], which was derived from INS-1 rat insulinoma cells [32] and was a gift from C. B. Newgard (Duke University, Durham, NC, USA). Insulin secretion and content assays were performed as previously described [17, 25, 31].
Measurement of malate and citrate by gas chromatography/mass spectrometry
Cytosolic and mitochondrial malate and citrate levels were measured relative to an added [1C13]malate or [2H4]citrate as internal standards (IsoTec, Toronto, ON, Canada) by gas chromatography/mass spectrometry, as previously described [17]. In brief, at the end of the secretion assay, cells were treated with saponin (80 μg/ml) to selectively permeabilise the plasma membrane of cells, followed by centrifugation [17, 33, 34]. The supernatant fraction represented the cytosolic fraction and the cell pellet representing the mitochondrial fraction [17]. For more details, see Electronic supplementary material (ESM), Measurement of malate.
siRNA duplexes and construction of the adenovirus siDIC recombinant adenovirus
Three siRNA duplexes were tested against the rat Dic (accession number BC081734). The siRNA sequences and transfection protocol can be found in the ESM (siRNA duplexes). Experiments were performed 3 days after duplex transfection.
The siRNA sequences for Dic (DIC-1–3) and scrambled control siRNA (siControl) were used to prepare recombinant adenoviruses by previously described methods [17, 25]. The siRNA adenoviruses were purified using a kit (Adeno-X Purification Kit; BD Biosciences, Clontech, Palo Alto, CA, USA) and virus titre was estimated by measuring absorbance at 260 nm.
Real-time PCR analysis of Dic mRNA expression
RNA was isolated from 832/13 cells using a kit (Aurum RNA Mini kit; BioRad, Hercules, CA, USA) and from primary rat islets using MicroRNA kit (Qiagen, Valencia, CA, USA). RNA was reverse-transcribed using a kit (iScript cDNA synthesis kit; BioRad). Dic (also known as Slc25a10) mRNA levels were detected by real-time PCR as previously described [17, 25], using prevalidated Dic and 18S RNA-specific fluorescent probes obtained from Applied Biosystems (Foster City, CA, USA).
Cell viability assay
Cell viability was assessed either by the cytotoxicity assay (SIGMA, Oakville, ON, Canada) or the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) mitochondrial dye method.
DIC immunoblot
DIC immunoblots were performed as described in the ESM (DIC immunoblot).
Isolation of mitochondria and measurement of mitochondrial malate transport
Mitochondria were prepared and assayed for malate transport from control (untreated), siControl and siDIC-2-treated 832/13 cells as previously described [35]; for more detailed protocol, see corresponding section of ESM. Malate exported from cell mitochondria was assayed enzymatically with alkali enhanced fluorescence of NAD(P)(H) [35, 36] (details, see ESM).
Glucose utilisation, glucose oxidation and glucose incorporation into fatty acids
Samples were processed for measurement of glucose utilisation, glucose oxidation and glucose incorporation into fatty acids as previously described [17, 25] and in ESM (Glucose utilisation, Glucose oxidation). Briefly, 832/13 cells were preincubated for 2 h in KRB secretion buffer with low glucose (2 mmol/l). After the preincubation period, 832/13 cells were cultured in the presence of tracer.
ADP and ATP determination
Cells (832/13 cells) were pretreated for 2 h in KRB with 2.8 mmol/l glucose and then incubated for 2 h in KRB with 2.8 or 16.7 mmol/l glucose. At the end of the experiment, cells were collected and snap-frozen in dry ice/ethanol. The cell pellets were stored at −80°C until assayed. ATP and ADP content was determined as described [17, 37].
NADPH and NADP+ assays
Cells (832/13 cells) were pretreated for 2 h in KRB with 2.8 mmol/l glucose and then incubated for 2 h at 2.8 or 16.7 mmol/l glucose. At the end of the experiment, cells were collected and snap-frozen in dry ice/ethanol. The cell pellets were stored at −80°C until assayed. NADP+ and NADPH levels were measured as described [17, 23].
Islet isolation and insulin secretion
Islets were removed from adult male Sprague–Dawley rats weighing approximately 250 to 300 g, and cultured as previously described [17, 25] and detailed in the corresponding ESM section. Insulin assay and insulin content were measured as previously described [17, 25, 37] and in ESM (Islet isolation). Islet butylmalonate studies were performed 24 h after islet isolation. For these studies two groups of islets were used: control (untreated islets) or islets treated with butylmalonate. Butylmalonate was only added during the insulin secretion assay. For the adenovirus studies we used three groups of islets: control (untreated), Ad-siControl and adenovirus siDIC-2 (Ad-siDIC-2). For each experimental group, 100 small to medium-sized islets were either untreated or treated with 1000 particles/ml of adenovirus overnight (final volume 2 ml), washed with growth media and cultured for 2 days. Compared with the untreated group of islets, there were no differences in islet cell viability between islets treated with Ad-siControl and those treated with Ad-siDIC-2.
For high glucose or palmitate treatments, islets were preincubated for 24 h in islet culture medium (described above) and then transferred to control medium (culture medium with 5% FBS [vol./vol.] and 0.25% BSA [0.25%] without NEFA [Sigma]), control medium with high glucose (20 mmol/l) or palmitate medium (control medium with 0.4 mmol/l palmitate) for 48 h. After 48 h, islet cDNA was prepared and Dic expression assessed by real-time PCR as described above.
Statistics
Statistical significance was assessed by Student’s t test or by one-way or two-way ANOVA for repeated measures followed by multiple Bonferroni comparisons. All data are expressed as means ± SEM. All experimental protocols used in this study were approved by the Research Ethics Board of the University of Waterloo.
Results
The DIC inhibitor butylmalonate dose-dependently inhibits GSIS in 832/13 cells
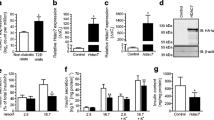
Butylmalonate dose-dependently inhibited insulin release in response to glucose in the INS-1-derived 832/13 cells (Fig. 1a). Butylmalonate (2 mmol/l) significantly inhibited insulin secretion stimulated by glucose + KCl (16.7 mmol/l glucose + 30 mmol/l KCl) by 8.8 ± 3% as compared with untreated cells (Fig. 1a). To stimulate insulin secretion independently of glucose metabolism, we used 30 mmol/l KCl in these studies. Butylmalonate (2 mmol/l) did not affect basal insulin secretion or GSIS (Fig. 1a). However, addition of 5 mmol/l butylmalonate resulted in a significant inhibition of insulin release stimulated both by glucose and by glucose + KCl (47 ± 3% and 49 ± 4% respectively; Fig. 1a), whereas 10 mmol/l butylmalonate further suppressed both of the above by 62 ± 2% and 81 ± 1%, respectively. KCl may be expected not to restore GSIS, if the role of DIC is independent of the glucose-stimulated rise of Ca2+ and is dependent on some other mitochondria-derived signalling molecule that modulates insulin release. At 10 mmol/l butylmalonate basal insulin release was significantly elevated (p < 0.001).
The DIC inhibitor butylmalonate (BM) dose-dependently inhibits GSIS, and inhibits mitochondrial malate and citrate transport in 832/13 cells. a GSIS in response to 2, 5 and 10 mmol/l butylmalonate and glucose as labelled (n = 4), and (b) malate and (c) citrate transport in response to glucose stimulation (n = 5 for both) as labelled: LG, low glucose (2.8 mmol/l); HG, high glucose (16.7 mmol/l); HG + KCl, high glucose plus KCl (16.7 mmol/l glucose, 30 mmol/l KCl). Cytosolic and mitochondrial malate and citrate were separated as described in Methods section. *p < 0.05 and ***p < 0.001 for HG control vs HG butylmalonate; † p < 0.05 and ††† p < 0.001 for HG + KCl control vs HG + KCl plus butylmalonate; ‡‡‡ p < 0.001 for LG control vs LG butylmalonate
Butylmalonate effects on cytosolic and mitochondrial malate and citrate levels
Cytosolic and mitochondrial malate and citrate concentrations were measured in 832/13 cells cultured at either low or high glucose concentrations in the absence or presence of the specific DIC inhibitor butylmalonate. As expected, incubation of 832/13 cells in the presence of 5 mmol/l butylmalonate significantly reduced glucose-stimulated mitochondrial export of malate by 39 ± 5% (Fig. 1b). In contrast, butylmalonate-mediated inhibition of DIC did not cause any significant changes in mitochondrial malate levels of 832/13 cells incubated at low or high glucose concentrations. In addition, 5 mmol/l butylmalonate significantly reduced glucose-stimulated mitochondrial citrate export into the cytosolic compartment by 35 ± 4% without affecting mitochondrial citrate levels (Fig. 1c). The lack of an increase in mitochondrial malate levels was not unexpected as levels of tricarboxylic acid (TCA) intermediates are highly regulated. These results suggest that cytosolic malate provided by DIC is essential for export of mitochondrial citrate by CIC (CIC transports citrate in exchange for malate [17]).
The effects of butylmalonate on insulin secretion did not affect glucose-stimulated changes in the ATP/ADP ratio (data not shown) and insulin content from 832/13 cells (control 8934 ± 145; butylmalonate 2 mmol/l 8780 ± 231; butylmalonate 10 mmol/l 8611 ± 579 μU insulin/mg protein). None of the effects of butylmalonate could be ascribed to cytotoxicity, as treatment of 832/13 cells for 3 h with 10 mmol/l butylmalonate had no effect on cell viability as assessed by ToxiLight cytotoxicity assay or the MTS mitochondrial dye method (data not shown).
Transfection-based siRNA-mediated suppression of Dic expression inhibits GSIS
Because we cannot rule out the possibility that butylmalonate targets other proteins besides DIC, we next used siRNA technology to ensure that specific targeting of DIC had occurred and tried to repeat some of our earlier findings done with the pharmacological inhibitor. To this end, 832/13 cells were transfected with three siRNA duplexes directed against different regions of Dic mRNA or a control, non-specific siRNA duplex (siControl). The three DIC-specific siRNA duplexes reduced the number of Dic gene transcripts by approximately 48% to 92% (Fig. 2a), causing a reduction in GSIS (5–69%) that was proportionate to the degree of Dic knockdown by each of the siRNA duplexes (Fig. 2b).
Three different siRNA duplexes against Dic (DIC-1–3) inhibited GSIS in 832/13 cells. a The effects of the three siRNA duplexes on Dic gene expression (n = 4) and (b) on GSIS (n = 4) with glucose stimulation as labelled: LG, low glucose (2.8 mmol/l); HG, high glucose (16.7 mmol/l). Control cells (b) were untreated; siControl cells were treated with an siRNA that has no known target. c Western blot data (siCon, cells treated with siControl; siDIC-2, cells treated with siDIC-2) (n = 6). d Effects on KCl-stimulated insulin secretion (n = 6), with glucose stimulation as labelled. HG + KCl, high glucose plus KCl (16.7 mmol/l glucose, 30 mmol/l KCl). **p < 0.01 and ***p < 0.001 for HG control vs HG siDIC-2; ††† p < 0.001 for HG + KCl control vs HG + KCl plus siDIC-2
To further examine the role of DIC on GSIS, one of the highly efficient siRNA duplexes (siDIC-2) was used as a tool to suppress DIC in our subsequent studies. The siDIC-2 duplex reduced Dic mRNA by 76 ± 4% and protein levels by 70 ± 8% (Fig. 2c). These effects on gene and protein levels caused a 64 ± 3% reduction in GSIS and a 33 ± 6% reduction in insulin release stimulated by glucose + KCl (Fig. 2d). As with the butylmalonate studies, GSIS was not restored with KCl in siDIC-2-treated cells suggesting that DIC may play a role in generating a unique mitochondria-derived signalling molecule.
Effects of butylmalonate and siDIC-2 on malate transport from isolated mitochondria
Mitochondria were isolated from 832/13 cells and used to assess the effects of butylmalonate and siDIC-2 on pyruvate-stimulated malate export from isolated mitochondria. Mitochondria were treated with no substrate (background malate export without a stimulus) or with pyruvate. Pyruvate enters the mitochondrial TCA cycle and is converted to malate. No significant differences were seen for pyruvate-stimulated malate transport in control mitochondria and mitochondria treated with the control siRNA (siControl; Fig. 3). Butylmalonate dose-dependently inhibited pyruvate-stimulated malate transport as compared with control mitochondria (Fig. 3). Mitochondria treated with siDIC-2 also inhibited pyruvate-stimulated malate transport from isolated mitochondria as compared with control mitochondria (Fig. 3). These results suggest that pharmacological or molecular biology-based methods of inhibiting DIC reduce malate transport in isolated mitochondria.
Malate transport measured in isolated mitochondria from 832/13 cells. Control mitochondria were incubated with no pyruvate (no substrate-treated mitochondria). Mitochondria treated with pyruvate (a TCA substrate that generates mitochondrial malate) were untreated or treated with siControl, siDIC-2 or butylmalonate (BM, n = 4). **p < 0.01 and ***p < 0.001 for control vs treatment
Metabolic effects of DIC suppression
We next examined several potential metabolic mechanisms by which DIC suppression may inhibit GSIS. Treatment of 832/13 cells with siDIC-2 did not affect glucose utilisation (Fig. 4a) or glucose oxidation (Fig. 4b) relative to untreated (control) or siControl treatment, but did significantly reduce incorporation of radiolabelled glucose into fatty acids by 26 ± 5% (Fig. 4c). Similarly to findings for the pharmacological DIC inhibitor, ATP, ADP or ATP:ADP levels were not significantly altered by siDIC-2-mediated suppression of DIC activity (Fig. 5).
Metabolic effects of siDIC-2 in 832/13 cells. a Glycolytic rate (n = 4), (b) glucose oxidation (n = 4) and (c) [U-14C]glucose incorporation into fatty acids (n = 4) with glucose stimulation as labelled: LG, low glucose (2.8 mmol/l); HG, high glucose (16.7 mmol/l). *p < 0.05 for HG siControl vs HG siDIC-2
We have previously shown that the mitochondrial export of citrate/isocitrate regulates GSIS via the ICDc-mediated production of cytosolic NADPH [23]. It has been proposed that the pyruvate/isocitrate cycle depends on a cytosolic source of malate for efficient mitochondrial export of citrate/isocitrate via CIC [17]. Consistent with this hypothesis, siDIC-2 treatment of 832/13 cells resulted in a significant reduction of glucose-stimulated increments of the NADPH/NADP+ ratio (Fig. 6).
The effects of siDIC-2 on glucose-stimulated alterations in (a) NADPH (white bars) and NADP+ (black bars), and (b) NADPH/NADP+ ratio in 832/13 cells (n = 6). Glucose stimulation (a) as labelled: LG, low glucose (2.8 mmol/l); HG, high glucose (16.7 mmol/l). b White bars, low glucose; black bars, high glucose. *p < 0.05 for NADPH HG siControl vs NADPH HG siDIC-2; † p < 0.05 for LG siControl vs LG siDIC-2
Adenovirus-mediated delivery of a Dic-specific siRNA inhibits insulin release in 832/13 cells
To further investigate the effects of Dic knockdown in isolated rat islets, we constructed three recombinant adenoviruses (Ad-siDIC-1–3) containing the same siRNA sequences as described in ESM for siDIC-1–3. Treatment of 832/13 cells with Ad-siDIC-2 resulted in a significant decrease in Dic mRNA by 87 ± 4% and GSIS as compared with cells treated with Ad-siControl. Similar results were found for Ad-siDIC-1 (76 ± 5% reduction in GSIS). Consistent with the effects of siDIC-3, the Ad-siDIC-3 did not reduce DIC expression levels (42 ± 5% reduction) enough to significantly inhibit GSIS. These results confirm the siRNA duplex experiments in 832/13 cells and suggest that DIC plays an important role in insulin secretion and DIC is also a major conduit for malate export from mitochondria.
The DIC inhibitor butylmalonate inhibits GSIS in isolated rat islets
We next sought to confirm the effects of butylmalonate in 832/13 cells in primary rat islets. In static incubation experiments, addition of 2 mmol/l butylmalonate to rat islets inhibited insulin secretion in response to 16.7 mmol/l glucose by 29 ± 8% as compared with islets incubated without the drug (Fig. 7a). In rat islets, butylmalonate (5 mmol/l) inhibited insulin release by 39 ± 7% in response to 16.7 mmol/l glucose. These experiments revealed that butylmalonate inhibited insulin secretion in response to 16.7 mmol/l glucose. The inhibitory effects of butylmalonate could be reversed with dimethyl malate (Fig. 7b). Dimethyl malate is a membrane permeable analogue of malate and a known activator of pyruvate cycling and NADPH production [15, 38].
The effects of (a) butylmalonate (BM) (n = 6), (b) butylmalonate and rescue with dimethyl malate (DMM), and (c) Ad-siDIC-2 (n = 5) on insulin secretion in isolated rat islets, with glucose stimulation as labelled: LG, low glucose (2.8 mmol/l); HG, high glucose (16.7 mmol/l); HG + KCl, high glucose plus KCl (16.7 mmol/l glucose, 30 mmol/l KCl). *p < 0.05, **p < 0.01 and ***p < 0.001 for HG Ad-siControl vs HG butylmalonate or Ad-siDIC-2; †p < 0.05 for HG + KCl Ad-siControl vs HG + KCl plus Ad-siDIC-2
An siRNA adenovirus expressing an siRNA against Dic inhibits GSIS in isolated rat islets
An adenovirus that expresses an siRNA against Dic (Ad-siDIC-2) reduced Dic mRNA levels by 59 ± 9% (p < 0.01) as compared with Ad-siControl-treated islets. In isolated rat islets, this amount of Dic knockdown resulted in 36 ± 5% inhibition of GSIS and 33 ± 4% inhibition of insulin secretion stimulated by glucose + KCl (Fig. 7c), a finding consistent with those for 832/13 cells. There was no statistical significance for GSIS between untreated control islets and Ad-siControl-treated islets (results not shown). Ad-siDIC-2 treatment did not affect islet insulin content relative to Ad-siControl-treated islets (results not shown). Taken together, these results show that the observed effects of DIC suppression in INS-1 832/13 cells can be repeated in primary cultured isolated rat islets.
Effects of palmitate and high glucose on Dic gene expression
Treatment of islets with 0.4 mmol/l palmitate for 48 h, but not with 20 mmol/l glucose, reduced Dic expression as compared with control cells (Fig. 8). These results suggest that diet-induced obesity may lead to a reduction of DIC in islets.
Schematic diagram showing the potential role of DIC in GSIS. This study supports a role for DIC in insulin secretion. DIC may provide cytosolic malate that is required for CIC to export citrate or isocitrate from the mitochondrial matrix. This process fits into a bigger theme on nutrient-regulated insulin secretion involving pyruvate cycling via the pyruvate–isocitrate pathway (grey). For the pyruvate–isocitrate pathway, oxaloacetate, the product of pyruvate carboxylase, is converted to citrate and isocitrate. Both intermediates are capable of exiting the mitochondria via the CIC carrier in exchange for cytosolic malate. Cytosolic aconitase catalyses conversion of citrate to isocitrate and, in turn, isocitrate can be converted to α-ketoglutarate via ICDc. α-Ketoglutarate may enter the mitochondria for conversion to malate by tricarboxylic acid cycle enzymes, with subsequent conversion to pyruvate by malic enzyme (MEm), thus completing the pyruvate cycle. The cycling of pyruvate via the pyruvate–isocitrate pathway probably results in generation of signalling molecule(s) that modulate insulin secretion. Two possible signalling molecules that can modulate insulin release are NADPH and α-ketoglutarate. OGC, 2-oxoglutarate carrier
Discussion
In our previous studies, we have demonstrated that CIC-mediated export of citrate/isocitrate [17] and the cytosolic production of NADPH by cytosolic NADP+-dependent isocitrate dehydrogenase [23] plays a critical role in GSIS. Here, we examined the role of DIC in cytosolic NADPH production and GSIS. We hypothesised that DIC would be required to provide sufficient amounts of cytosolic malate, which can serve as a counter substrate in CIC-mediated translocation of mitochondrial citrate/isocitrate to the cytosol (Fig. 9). These studies demonstrate that DIC plays an important role in regulating insulin release in response to glucose. Inhibition of DIC activity by butylmalonate resulted in a reduction of cytosolic malate and citrate levels. Butylmalonate and the use of siRNAs against Dic both resulted in inhibition of KCl-stimulated insulin secretion, suggesting that the effects of DIC on insulin secretion are independent of Ca2+-mediated exocytosis. Knockdown of Dic caused a significant lowering of NADPH/NADP+ ratio, the inhibitory effects of butylmalonate being reversible by dimethyl malate, a known activator of pyruvate cycling that can promote NADPH production [15, 38]. These findings support the concept that the ability of glucose to stimulate an increase in cytosolic malate levels plays an important role in the control of GSIS. Moreover, since Dic knockdown had no effect on glycolytic flux, glucose oxidation or ATP/ADP ratio, this suggests that no non-specific or global effect of Dic knockdown on beta cell glucose metabolism occurred, and no change in cell viability.
Several theories on alternative signalling molecules involved in GSIS revolve around a key concept in metabolism called anaplerosis. A role for anaplerosis in insulin release has been suggested, as approximately 40% to 50% of pyruvate entering mitochondrial pathways at high glucose does so via the key anaplerotic enzyme, pyruvate carboxylase [39–41]. Several anaplerosis-derived metabolites have been suggested to act as important signalling molecules regulating insulin secretion; these include glutamate, malonyl-CoA, long-chain-acyl-CoA, α-ketoglutarate and NADPH [15, 41–44].
Malonyl-CoA and long-chain-acyl-CoA have been proposed to play an important role in GSIS. Glucose-dependent production of malonyl-CoA and long-chain-acyl-CoA depends on pyruvate carboxylase-mediated anaplerotic elevation of cytosolic citrate levels [45, 46]. Citrate can be processed into malonyl-CoA and subsequently to long-chain-acyl-CoA. In addition, malonyl-CoA is a potent inhibitor of carnitine palmitoyltransferase I [47], an action that could divert long-chain-acyl-CoA away from oxidation in the mitochondria towards accumulation in the cytosol [46]. All these steps lead to an elevation of cytosolic malonyl-CoA and long-chain-acyl-CoA. This model has it pros and cons, which have been reviewed extensively elsewhere [12, 48]. Our recent studies [25, 38], as well as others [49–51] have now shown a consistent lack of evidence for a direct role of malonyl-CoA in regulation of GSIS, whereas its potential role in lipid-mediated potentiation of GSIS remains a possibility. Our results showing that inhibition of DIC leads to a reduction of glucose incorporation into lipid support this hypothesis.
NADPH has also been suggested to play an important role as a metabolic messenger regulating insulin release [15, 17, 19, 22–24, 52]. A key piece of evidence supporting a role for NADPH in insulin release is that addition of NADPH to patch-clamped beta cells stimulated exocytosis, whereas NADH had no effect. These studies also suggest that the NADPH/NADP+ ratio may be the relevant signal, since addition of NADP+ reversed the stimulatory effect obtained with NADPH alone [52]. We have also provided additional support for a role of NADPH from studies showing that suppression of ICDc (a key NADPH-producing enzyme) by adenovirus-mediated delivery of an siRNA construct caused a coordinate reduction in the NAPDH/NADP+ ratio and inhibition of GSIS [23]. We have also shown that suppression of CIC inhibits NADPH production and GSIS [17]. Our current data support the concept that DIC provides a cytosolic source of malate that could be used as a counter ion for citrate/isocitrate export by CIC for use by ICDc to produce NADPH. Our current findings also support a role for NADPH in GSIS, as we found that siRNA-mediated knockdown of Dic also significantly reduced the increment in NADPH/NADP+ ratio at high glucose levels. A major question in the field is whether NADPH is involved in insulin secretion, and if so, what is it targeting? Voltage-gated K+ channels are one potential target of NADPH [53]; other potential targets have been reviewed previously [12, 42].
Other by-products of anaplerosis and pyruvate cycling could also play a critical role in regulating insulin secretion. For example, a signalling role for cytosolic α-ketoglutarate or an intermediate produced from its further metabolism has been suggested [54]. Our findings from the present study, looking at the role of DIC in insulin secretion, supports this possibility. The 2-oxoglutarate carrier transports α-ketoglutarate out of mitochondria in exchange for cytosolic malate [55]. We have recently shown that dimethyl α-ketoglutarate is able to stimulate insulin secretion and that knockdown of the gene encoding α-ketoglutarate carrier caused a significant impairment of this response [55]. We have also shown that siRNA-mediated knockdown of Icdc which metabolises isocitrate to α-ketoglutarate, also inhibits insulin release [23]. These two studies suggest that either transport of or generation of cytosolic α-ketoglutarate is a key event in control of insulin secretion.
It remains unclear exactly how α-ketoglutarate regulates insulin release, but it could regulate beta cell insulin secretion by serving as a substrate for α-ketoglutarate hydroxylases. α-Ketoglutarate hydroxylases modify proline residues on hypoxia-inducible transcription factors and possibly other substrates, promoting their degradation and inactivation [56]. Support for this concept comes from studies showing that acute inhibition of α-ketoglutarate hydroxylases causes impairment of insulin secretion [57].
In summary, the current study investigates the role of glucose-stimulated export of mitochondrial malate in the production of cytosolic NADPH and in the regulation of insulin secretion. We have shown that the DIC inhibitor, butylmalonate, and siRNA-mediated knockdown of Dic inhibited GSIS from INS-1-derived 832/13 cells and isolated rat islets. The effects of DIC inhibition could be reversed by dimethyl malate, which restores cytosolic malate levels, and promotes pyruvate cycling and NADPH production. These results support a role for DIC in insulin secretion, consisting possibly of provision of cytosolic malate, which may serve as a counter substrate for CIC-mediated export of citrate/isocitrate from the TCA cycle. In combination with our earlier work on CIC [17] and ICDc [23], the present study on the role of DIC suggests a novel mechanism in regulation of insulin release that involves pyruvate cycling via the pyruvate/isocitrate pathway (Fig. 8b).
Abbreviations
- Ad-siDIC-2:
-
Adenovirus siDIC-2
- CIC:
-
Citrate/isocitrate carrier
- DIC:
-
Dicarboxylate carrier
- GSIS:
-
Glucose-stimulated insulin secretion
- ICDc:
-
Cytosolic NADP+-dependent isocitrate dehydrogenase
- MTS:
-
3-(4,5-Dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
- siControl:
-
Scrambled control siRNA
- siDIC:
-
siRNA targeted against Dic
- siRNA:
-
Small interfering RNA
- TCA:
-
Tricarboxylic acid
References
Ashcroft FM, Rorsman P (1989) Electrophysiology of the pancreatic beta-cell. Prog Biophys Mol Biol 54:87–143
Henquin JC (2004) Pathways in beta-cell stimulus-secretion coupling as targets for therapeutic insulin secretagogues. Diabetes 53(Suppl 3):S48–S58
Gembal M, Gilon P, Henquin JC (1992) Evidence that glucose can control insulin release independently from its action on ATP-sensitive K+ channels in mouse B cells. J Clin Invest 89:1288–1295
Henquin JC, Ravier MA, Nenquin M, Jonas JC, Gilon P (2003) Hierarchy of the beta-cell signals controlling insulin secretion. Eur J Clin Invest 33:742–750
Nenquin M, Szollosi A, Guilar-Bryan L, Bryan J, Henquin JC (2004) Both triggering and amplifying pathways contribute to fuel-induced insulin secretion in the absence of sulfonylurea receptor-1 in pancreatic beta-cells. J Biol Chem 279:32316–32324
Sato Y, Aizawa T, Komatsu M, Okada N, Yamada T (1992) Dual functional role of membrane depolarization/Ca2+ influx in rat pancreatic B-cell. Diabetes 41:438–443
Szollosi A, Nenquin M, Henquin JC (2010) Pharmacological stimulation and inhibition of insulin secretion in mouse islets lacking ATP-sensitive K(+) channels. Br J Pharmacol 159:669–677
Ravier MA, Nenquin M, Miki T, Seino S, Henquin JC (2009) Glucose controls cytosolic Ca2+ and insulin secretion in mouse islets lacking adenosine triphosphate-sensitive K+ channels owing to a knockout of the pore-forming subunit Kir6.2. Endocrinology 150:33–45
Quoix N, Cheng-Xue R, Mattart L et al (2009) Glucose and pharmacological modulators of ATP-sensitive K+ channels control [Ca2+]c by different mechanisms in isolated mouse alpha-cells. Diabetes 58:412–421
Henquin JC, Nenquin M, Ravier MA, Szollosi A (2009) Shortcomings of current models of glucose-induced insulin secretion. Diabetes Obes Metab 11(Suppl 4):168–179
Henquin JC (2009) Regulation of insulin secretion: a matter of phase control and amplitude modulation. Diabetologia 52:739–751
Jensen MV, Joseph JW, Ronnebaum SM, Burgess SC, Sherry AD, Newgard CB (2008) Metabolic cycling in control of glucose-stimulated insulin secretion. Am J Physiol Endocrinol Metab 295:E1287–E1297
Hasan NM, Longacre MJ, Stoker SW et al (2008) Impaired anaplerosis and insulin secretion in insulinoma cells caused by small interfering RNA-mediated suppression of pyruvate carboxylase. J Biol Chem 283:28048–28059
MacDonald MJ, Stoker SW, Hasan NM (2008) Anaplerosis from glucose, alpha-ketoisocaproate, and pyruvate in pancreatic islets, INS-1 cells and liver mitochondria. Mol Cell Biochem 313:195–202
Lu D, Mulder H, Zhao P et al (2002) 13C NMR isotopomer analysis reveals a connection between pyruvate cycling and glucose-stimulated insulin secretion (GSIS). Proc Natl Acad Sci USA 99:2708–2713
Maechler P, Antinozzi PA, Wollheim CB (2000) Modulation of glutamate generation in mitochondria affects hormone secretion in INS-1E beta cells. IUBMB Life 50:27–31
Joseph JW, Jensen MV, Ilkayeva O et al (2006) The mitochondrial citrate/isocitrate carrier plays a regulatory role in glucose-stimulated insulin secretion. J Biol Chem 281:35624–35632
Agascioglu E, Giroix MH, Malaisse WJ, Sener A (2006) Adenine nucleotide pattern in rat pancreatic islets exposed to nutrient secretagogues. Endocr 29:325–329
Jensen MV, Joseph JW, Ilkayeva O et al (2006) Compensatory responses to pyruvate carboxylase suppression in islet beta-cells. Preservation of glucose-stimulated insulin secretion. J Biol Chem 281:22342–22351
Szollosi A, Nenquin M, Henquin JC (2007) Overnight culture unmasks glucose-induced insulin secretion in mouse islets lacking ATP-sensitive K+ channels by improving the triggering Ca2+ signal. J Biol Chem 282:14768–14776
Szollosi A, Nenquin M, Guilar-Bryan L, Bryan J, Henquin JC (2007) Glucose stimulates Ca2+ influx and insulin secretion in 2-week-old beta-cells lacking ATP-sensitive K+ channels. J Biol Chem 282:1747–1756
Palmieri F (2004) The mitochondrial transporter family (SLC25): physiological and pathological implications. Pflügers Arch Eur J Physiol 447:689–709
Ronnebaum SM, Ilkayeva O, Burgess SC et al (2006) A pyruvate cycling pathway involving cytosolic NADP-dependent isocitrate dehydrogenase regulates glucose-stimulated insulin secretion. J Biol Chem 281:30593–30602
Cline GW, Lepine RL, Papas KK, Kibbey RG, Shulman GI (2004) 13C NMR isotopomer analysis of anaplerotic pathways in INS-1 cells. J Biol Chem 279:44370–44375
Joseph JW, Odegaard ML, Ronnebaum SM et al (2007) Normal flux through ATP-citrate lyase or fatty acid synthase is not required for glucose-stimulated insulin secretion. J Biol Chem 282:31592–31600
Brown LJ, Longacre MJ, Hasan NM, Kendrick MA, Stoker SW, MacDonald MJ (2009) Chronic reduction of the cytosolic or NAD(P)-mitochondrial malic enzyme does not affect insulin secretion in a rat insulinoma cell line. J Biol Chem 284:35359–35367
Ronnebaum SM, Jensen MV, Hohmeier HE et al (2008) Silencing of cytosolic or mitochondrial isoforms of malic enzyme has no effect on glucose-stimulated insulin secretion from rodent islets. J Biol Chem 283:28909–28917
Xu J, Han J, Long YS et al (2008) Malic enzyme is present in mouse islets and modulates insulin secretion. Diabetologia 51:2281–2289
Pongratz RL, Kibbey RG, Shulman GI, Cline GW (2007) Cytosolic and mitochondrial malic enzyme isoforms differentially control insulin secretion. J Biol Chem 282:200–207
Guay C, Madiraju SR, Aumais A, Joly E, Prentki M (2007) A role for ATP-citrate lyase, malic enzyme, and pyruvate/citrate cycling in glucose-induced insulin secretion. J Biol Chem 282:35657–35665
Hohmeier HE, Mulder H, Chen G, Henkel-Rieger R, Prentki M, Newgard CB (2000) Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes 49:424–430
Asfari M, Janjic D, Meda P, Li G, Halban PA, Wollheim CB (1992) Establishment of 2-mercaptoethanol-dependent differentiated insulin-secreting cell lines. Endocrinology 130:167–178
Koshkin V, Bikopoulos G, Chan CB, Wheeler MB (2004) The characterization of mitochondrial permeability transition in clonal pancreatic beta-cells. Multiple modes and regulation. J Biol Chem 279:41368–41376
Civelek VN, Deeney JT, Shalosky NJ et al (1996) Regulation of pancreatic beta-cell mitochondrial metabolism: influence of Ca2+, substrate and ADP. Biochem J 318:615–621
MacDonald MJ (1995) Feasibility of a mitochondrial pyruvate malate shuttle in pancreatic islets. Further implication of cytosolic NADPH in insulin secretion. J Biol Chem 270:20051–20058
MacDonald MJ (1982) Evidence for the malate aspartate shuttle in pancreatic islets. Arch Biochem Biophys 213:643–649
Joseph JW, Koshkin V, Saleh MC et al (2004) Free fatty acid-induced beta-cell defects are dependent on uncoupling protein 2 expression. J Biol Chem 279:51049–51056
Ronnebaum SM, Joseph JW, Ilkayeva O et al (2008) Chronic suppression of acetyl-CoA carboxylase 1 in beta-cells impairs insulin secretion via inhibition of glucose rather than lipid metabolism. J Biol Chem 283:14248–14256
MacDonald MJ (1993) Estimates of glycolysis, pyruvate (de)carboxylation, pentose phosphate pathway, and methyl succinate metabolism in incapacitated pancreatic islets. Arch Biochem Biophys 305:205–214
Khan A, Ling ZC, Landau BR (1996) Quantifying the carboxylation of pyruvate in pancreatic islets. J Biol Chem 271:2539–2542
Schuit F, De Vos A, Farfari S et al (1997) Metabolic fate of glucose in purified islet cells. Glucose-regulated anaplerosis in beta cells. J Biol Chem 272:18572–18579
MacDonald PE, Joseph JW, Rorsman P (2005) Glucose-sensing mechanisms in pancreatic beta-cells. Philos Trans R Soc Lond B Biol Sci 360:2211–2225
MacDonald MJ, Fahien LA, Brown LJ, Hasan NM, Buss JD, Kendrick MA (2005) Perspective: emerging evidence for signaling roles of mitochondrial anaplerotic products in insulin secretion. Am J Physiol Endocrinol Metab 288:E1–E15
Deeney JT, Prentki M, Corkey BE (2000) Metabolic control of beta-cell function. Semin Cell Dev Biol 11:267–275
Corkey BE, Glennon MC, Chen KS, Deeney JT, Matschinsky FM, Prentki M (1989) A role for malonyl-CoA in glucose-stimulated insulin secretion from clonal pancreatic beta-cells. J Biol Chem 264:21608–21612
Prentki M, Vischer S, Glennon MC, Regazzi R, Deeney JT, Corkey BE (1992) Malonyl-CoA and long chain acyl-CoA esters as metabolic coupling factors in nutrient-induced insulin secretion. J Biol Chem 267:5802–5810
McGarry JD (2002) Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes 51:7–18
Prentki M, Murthy M Sr (2008) Glycerolipid metabolism and signaling in health and disease. Endocr Rev 29:647–676
Chakravarthy MV, Zhu Y, Lopez M et al (2007) Brain fatty acid synthase activates PPARalpha to maintain energy homeostasis. J Clin Invest 117:2539–2552
MacDonald MJ, Hasan NM, Longacre MJ (2008) Studies with leucine, beta-hydroxybutyrate and ATP citrate lyase-deficient beta cells support the acetoacetate pathway of insulin secretion. Biochim Biophys Acta 1780:966–972
MacDonald MJ, Smith AD III, Hasan NM, Sabat G, Fahien LA (2007) Feasibility of pathways for transfer of acyl groups from mitochondria to the cytosol to form short chain acyl-CoAs in the pancreatic beta cell. J Biol Chem 282:30596–30606
Ivarsson R, Quintens R, Dejonghe S et al (2005) Redox control of exocytosis: regulatory role of NADPH, thioredoxin, and glutaredoxin. Diabetes 54:2132–2142
MacDonald PE, Salapatek AM, Wheeler MB (2003) Temperature and redox state dependence of native Kv2.1 currents in rat pancreatic beta-cells. J. J Physiol 546:647–653
Rabaglia ME, Gray-Keller MP, Frey BL, Shortreed MR, Smith LM, Attie AD (2005) Alpha-ketoisocaproate-induced hypersecretion of insulin by islets from diabetes-susceptible mice. Am J Physiol Endocrinol Metab 289:E218–E224
Odegaard ML, Joseph JW, Jensen MV et al (2010) The mitochondrial 2-oxoglutarate carrier is part of a metabolic pathway that mediates glucose- and glutamine-stimulated insulin secretion. J Biol Chem 285:16530–16537
Ozer A, Bruick RK (2007) Non-heme dioxygenases: cellular sensors and regulators jelly rolled into one? Nat Chem Biol 3:144–153
Fallon MJ, MacDonald MJ (2008) Beta-cell alpha-ketoglutarate hydroxylases may acutely participate in insulin secretion. Metabolism 57:1148–1154
Acknowledgements
This work was supported by a Canadian Institute of Health Research grant (to J. W. Joseph). The authors would like to thank P. E. Scherer (UT Southwestern, Dallas, TX, USA) for providing the DIC antibody.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 86 kb)
Rights and permissions
About this article
Cite this article
Huypens, P., Pillai, R., Sheinin, T. et al. The dicarboxylate carrier plays a role in mitochondrial malate transport and in the regulation of glucose-stimulated insulin secretion from rat pancreatic beta cells. Diabetologia 54, 135–145 (2011). https://doi.org/10.1007/s00125-010-1923-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-010-1923-5