Summary
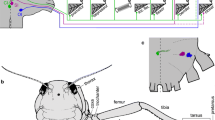
This study characterizes the fine structure of the “opercularis” muscles of selected frogs and salamanders (Genera: Hyla; Desmognathus; Ambystoma). The “opercularis” muscle originates on the shoulder girdle and inserts on the opercular plate in the fenestra ovalis of the otic capsule. Each of the three genera used exhibits one of the major gross dispositions of this muscle found in amphibians. In each case the “opercularis” muscle contains large numbers of tonic fibers: 80% in Hyla; 90% in Desmognathus; 45% in Ambystoma. These fibers correspond to the class-5 tonic fibers of Smith and Ovalle (1973). The remainder of the fibers in the “opercularis” correspond to those in the class-3 “phasic” of Smith and Ovalle. The muscle from which the “opercularis” is derived (levator scapulae in Hyla, cucullaris in Desmognathus) is comprised of fibers which correspond to the class-2 phasic fibers of Smith and Ovalle.
The fiber composition of the “opercularis” indicates that it is constructed to sustain contraction over long periods of time. This composition is supportive of the functional role in audition proposed for the muscle by Lombard and Straughan (1974). Evidence is presented that indicates that fiber size may be body size dependent and thus is an inappropriate criterion of fiber type identification.
Similar content being viewed by others
References
Baird, I.L.: Some aspects of the comparative anatomy and evolution of the inner ear in submammalian vertebrates. Brain Behav. Evol. 10, 11–36 (1974)
Baker, M.C.: The effects of severing the opercularis muscle on body orientation of the leopard frog, Rana pipiens. Copeia 1969, 613–616 (1969)
Capranica, R.R., Frishkopf, L.S., Nevo, E.: Encoding of geographic dialects in the auditory system of the cricket frog. Science 182, 1272–1275 (1973)
Capranica, R.R., Moffat, A.J.M.: Selectivity of the peripheral auditory system of spadefoot toads (Scaphiopus couchi) for sounds of biological significance. J. comp. Physiol. 100, 231–249 (1975)
Dunn, E.R.: The “opercularis” muscle of salamanders. J. Morph. 69, 207–216 (1941)
Eiselt, J.: Der Musculus opercularis und die mittlere Ohrsphäre der Amphibien. Arch. Naturgesch. 10, 179–270 (1941)
Emerson, S.B.: The fossorial frog adaptive zone: a study of convergence and parallelism in the Anura. Ph. D. Dissertation, University of Southern California (1970)
Gilly, W.F.: Slow fibers in the frog cruralis muscle. Tissue and Cell 7, 203–210 (1975)
Gradwell, N., Walcott, B.: Dual functional and structural properties of the interhyoideus muscle of the bullfrog tadpole (Rana catesbeiana). J. exp. Zool. 176, 193–218 (1971)
Hess, A.: The structure of vertebrate slow and twitch muscle fibers. Invest. Opthal. 6, 217–228 (1967)
Hess, A.: Vertebrate slow muscle fibers. Physiol. Rev. 50, 40–62 (1970)
Kingsbury, B.F., Reed, H.D.: The columella auris in Amphibia. J. Morph. 20, 549–628 (1909)
Kuffler, S.W., Vaughan Williams, E.M.: Small-nerve junctional potentials. The distribution of small motor nerves to frog skeletal muscle, and the frog membrane characteristics of the fibers they innervate. J. Physiol. (Lond.) 121, 289–317 (1953)
Loftus-Hills, J.J., Johnstone, B.M.: Auditory function, communication, and brain-evoked response in anuran amphibians. J. acoust. Soc. Amer. 47, 1131–1138 (1970)
Lombard, R.E.: A comparative morphological analysis of the salamander inner ear. Ph. D. Dissertation, University of Chicago (1971)
Lombard, R.E.: Comparative morphology of the inner ear in salamanders (Caudata: Amphibia). Contributions to Vertebrate Evolution, Vol. 2 (in press)
Lombard, R.E., Straughan, I.R.: Functional aspects of anuran middle ear structures. J. exp. Biol. 61, 71–93 (1974)
Lombard, R.E., Wake, D.B.: Tongue evolution in the lungless salamanders, family Plethodontidae. I. Introduction, theory and a general model of dynamics. J. Morph. 165, 1–22 (1976)
Luft, J.H.: Improvements in epoxy resin embedding methods. J. biophys. biochem. Cytol. 9, 409–414 (1961)
Monath, T.: The opercular apparatus of salamanders. J. Morph. 116, 149–170 (1965)
Nasledov, G.A., Federov, V.V.: On “transitional” fibers in skeletal musculature of frog [in Russian]. Arkh. Anat. Hist. Embriol. 8, 72–76 (1965)
Page, S.G.: Comparison of the fine structures of frog slow and twitch muscle fibers. J. Cell Biol. 26, 477–497 (1965)
Peachey, L.D., Huxley, A.F.: Structural identification of twitch and slow striated muscle fibers of the frog. J. Cell Biol. 13, 177–180 (1962)
Reynolds, E.S.: The use of lead citrate at high pH as an electron opaque stain in electron microscopy. J. Cell Biol. 17, 208–212 (1963)
Sedra, S.N., Michael, M.I.: The ontogenesis of the sound conducting apparatus of the Egyptian toad Bufo regularis Reuss, with a review of this apparatus in the salientia. J. Morph. 104, 359–373 (1959)
Smith, R.S., Ovalle, W.K., Jr.: Varieties of fast and slow extrafusal muscle fibers in amphibian hind limb muscles. J. Anat. (Lond.) 116, 1–24 (1973)
Steward, J.W.: The tailed amphibians of Europe, pp. 42–45. New York: Taplinger Publishing Co. 1969
Straughan, I.R.: An analysis of the mechanisms of mating call discrimination in the frogs Hyla regilla and H. cadaverina. Copeia 1975, 415–424 (1975)
Trump, B.F., Smuckler, E.A., Benditt, E.P.: A method for staining epoxy sections for light microscopy. J. Ultrastruct. Res. 5, 343–348 (1961)
Wake, D.B.: Comparative osteology and evolution of the lungless salamanders, family Plethodontidae. Mem. So. Cal. Acad. Sci. 4, 1–111 (1966)
Wever, E.G.: The Caecilian ear. J. exp. Zool. 191, 63–72 (1975)
Wilder, I.W., Dunn, E.R.: The correlation of lunglessness in salamanders with a mountain brook habitat. Copeia 84, 62–68 (1920)
Author information
Authors and Affiliations
Additional information
Acknowledgements. We would like to thank Donald Fischman for his advice and help in the early phases of this research. Sharon Emerson and James Hopson read a draft of this manuscript and their comments were very helpful. Elaine Olson prepared much of the ultrastructural data for us and in so doing aided our efforts considerably. Various drafts were typed by Terri Deno and Joan Hives. Terri Deno typed the final manuscript. The research was funded by a grant from the Louis Block Bequest Fund of the University of Chicago and USPHS Grant CA 05493
The authors are in alphabetical order. The sequence does not imply seniority
Rights and permissions
About this article
Cite this article
Becker, R.P., Lombard, R.E. Structural correlates of function in the “opercularis” muscle of amphibians. Cell Tissue Res. 175, 499–522 (1977). https://doi.org/10.1007/BF00222415
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00222415