Abstract
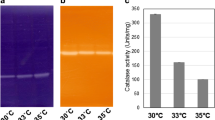
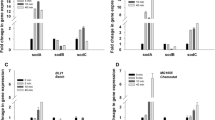
An increase in the superoxide dismutase (SOD) activity inStreptococcus lactis was observed when the cells were grown at increased oxygen partial pressures or exposed to hyperbaric oxygen tensions. The NADH-oxidase/NADH-peroxidase activities inS. lactis increased in galactose-grown cells when cultivated in air compared with N2/CO2. This effect did not occur when glucose was the carbon source; however, an increase in the activities of these enzymes was observed in oxygen atmosphere. The correlation between SOD, NADH-oxidase/NADH-peroxidase, and the metabolic pathways involved is discussed. The effect of manganese on the SOD activity is also considered.
Similar content being viewed by others
Literature Cited
Anders, R. F., Hogg D. M., Jago, G. R. 1970. Formation of hydrogen peroxide by group N streptococci and its effect on their growth and metabolism. Applied Microbiology19:608–612.
Asada, K., Yoshikawa, K., Takahashi, M., Maeda, Y., Enmanji, K. 1975. Superoxide dismutases from a bluegreen alga,Plectonema boryanum. Journal of Biological Chemistry250:2801–2807.
Britton, L., Malinowski, D. P., Fridovich, I. 1978. Superoxide dismutase and oxygen metabolism inStreptococcus faecalis and comparisons with other organisms. Journal of Bacteriology134:229–236.
Dolin, M. I. 1955. The DPNH-oxidizing enzymes ofStreptococcus faecalis. II. The enzymes utilizing oxygen, cytochrome c, peroxide and 2,6-dichlorophenol-indophenol or ferricyanide as oxidants. Archives of Biochemistry and Biophysics55:415–435.
Elstner, E. F. 1982. Oxygen activation and oxygen toxicity. Annual Review of Plant Physiology33:73–96.
Fridovich, I. 1975. Superoxide dismutases. Annual Review of Biochemistry44:147–159.
Fridovich, I. 1978. The biology of oxygen radicals. Science201:875–880.
Gottlieb, S. F. 1981. Oxygen toxicity in unicellular organisms, pp. 124–149. In: Gilbert, D. L., (ed.), Oxygen and living processes, New York: Springer-Verlag.
Götz, F., Elstner, E. F., Sedewitz, B., Lengfelder, E. 1980. Oxygen utilization byLactobacillus plantarum. II. Superoxide and superoxide dismutation. Archives of Microbiology125:215–220.
Gregory, E. M., Fridovich, I. 1973. Induction of superoxide dismutase by molecular oxygen. Journal of Bacteriology114:543–548.
Hansson, L., Häggström, M. H. 1983. Effects of growth conditions on superoxide dismutase and catalase activities inSaccharomyces cerevisiae var.ellipsoideus. Current Microbiology9:19–23.
Hassan, H. M., Fridovich, I. 1977. Physiological function of superoxide dismutase in glucose-limited chemostat cultures ofEscherichia coli. Journal of Bacteriology130:805–811.
Hassan, H. M., Fridovich, I. 1977. Regulation of superoxide dismutase synthesis inEscherichia coli: glucose effects. Journal of Bacteriology132:505–510.
Hassan, H. M., Fridovich, I. 1980. Superoxide dismutases: detoxication of free radical, pp. 311–332. In: Jacoby, W. B. (ed.), Enzymatic basis of detoxication, vol. 1, New York: Academic Press.
Hassan, H. M., Fridovich, I. 1982. Exacerbations of oxygen toxicity by redox active compounds, pp. 151–167. In: King, T. E., Mason, H. S., Morrison, M. (eds.) Oxidases and related redox systems: proceedings of the third international symposium. New York: Pergamon.
Johnson, M. J., Borkowski, J., Engblom, C. 1964. Steam sterilizable probes for dissolved oxygen measurement. Biotechnology and Bioengineering6:457–468.
Kirby, T., Blum, J., Kahane, I., Fridovich, I. 1980. Distinguishing between Mn-containing and Fe-containing superoxide dismutases in crude extracts of cells. Archives of Biochemistry and Biophysics201:551–555.
Korycka-Dahl, M., Richardson, T. 1980. Initiation of oxidative changes in foods. Journal of Dairy Science63:1181–1198.
Kulakova, S. M., Gogotov, I. N. 1982. Effect of oxygen and growth substrates on the activity of superoxide dismutase and catalase in microorganisms. Microbiology [English translation of Microbiologiya]51:16–21.
Lang, E., Lang, H. 1972. Spezifische Farbreaktion zum direkten Nachweis der Ameisensäure. Fresenius' Zeitschrift für Analytische Chemie260:8–10.
Lindmark, D. G., Paolella, P., Wood, N. P. 1969 The pyruvate formate-lyase system ofStreptococcus faecalis. I. Purification and properties of the formate-pyruvate exchange enzyme. Journal of Biological Chemistry244:3605–3612.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., Randall, R. J. 1951. Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry193:265–275.
McCord, J. M., Fridovich, I. 1969. Superoxide dismutase: an enzymic function for erythrocuprein (hemocuprein). Journal of Biological Chemistry244:6049–6055.
Moustafa, H. H., Collins, E. B. 1968. Rote of galactose or glucose-1-phosphate in preventing the lysis ofStreptococcus diacetilactis. Journal of Bacteriology95:592–602.
Petkau, A. 1978. Radiation protection by superoxide dismutase. Photochemistry and Photobiology28:765–774.
Petrone, W. F., English, D. K., Wong, K., McCord, J. M. 1980. Free radicals and inflammation: superoxide-dependent activation of a neutrophil chemotactic factor in plasma. Proceedings of the National Academy of Sciences of the United States of America77:1159–1163.
Pirt, S. J. 1975. Principles of microbe and cell cultivation, p. 125. Oxford: Blackwell Scientific Publications.
Ritchey, T. W., Seeley, H. W. Jr., 1976. Distribution of cytochrome-like respiration in Streptococci. Journal of General Microbiology93:195–203.
Steinman, H. M. 1982 Superoxide dismutases: protein chemistry and structure-function relationships, pp. 11–68. In: Oberley, L. W. (ed.), Superoxide dismutase, vol. 1 Florida: CRC.
Thomas, T. D., Ellwood, D. C., Longyear, V. M. C. 1979. Change from homo-to heterolactic fermentation byStreptococcus lactis resulting from glucose limitation in anaerobic chemostat cultures. Journal of Bacteriology138:109–117.
Walker, G. A., Kilgour, G. L. 1965. Pyridine nucleotide oxidizing enzymes ofLactobacillus casei. II. Oxidase and peroxidase. Archives of Biochemistry and Biophysics111:534–539.
Whitenbury, R. 1978. Biochemical characteristics ofStreptococcus species, pp. 51–69. In: Skinner, F. A., Quesnel, L. B. (eds.), Streptococci. London: Academic Press.
Yano, K., Nishie, H. 1978. Superoxide dismutase in facultatively anaerobic bacteria: enzyme levels in relation to growth conditions. Journal of General and Applied Microbiology24:333–339.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hansson, L., Häggström, M.H. Effects of growth conditions on the activities of superoxide dismutase and NADH-oxidase/NADH-peroxidase inStreptococcus lactis . Current Microbiology 10, 345–351 (1984). https://doi.org/10.1007/BF01626563
Issue Date:
DOI: https://doi.org/10.1007/BF01626563