Summary
-
1.
The reduction of the antibiotic abikoviromycin by complex metal hydrides and its hydrogenation in the presence of various catalysts have been studied.
-
2.
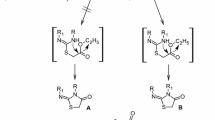
It has been established that abikoviromycin is 5-ethylidene-4,4a-epoxy-2,3,4,4a-tetrahydro-5H-1-pyrindine and has the (4S, 4aR, 5-1′E) configuration.
Similar content being viewed by others
Literature cited
H. Umezawa, T. Tazaki, and S. Fukuyama, Japan. Med. J.,4, 331 (1951); Chem. Abstr.,46, 7167 (1952).
Y. Sakagami, I. Yamaguchi, H. Yonehara, Y. Okimoto, S Yamanouchi, K. Takiguchi, and H. Sakai, J. Antibiotics, Ser. A,11, 6 (1958).
Y. Sakagami, R. Utahara, K. Yagishita, and H. Umezawa, J. Antibiotics, Ser. A,11, 231 (1958).
V. M. Roikhel' and N. A. Zeitlenok, Antibiotiki,14, 969 (1969).
A. I. Gurevich, M. N. Kolosov, V. G. Korobko, and V. B. Onoprienko, Tetrahedron Lett.,1968, 2209.
A. I. Gurevich, M. N. Kolosov, V. G. Korobko, V. D. Kuznetsov, and V. V. Onoprienko, Dokl. Akad. Nauk SSSR,182, 828 (1968).
A. I. Scott, Interpretation of the Ultraviolet Spectra of Natural Products, Pergamon Press, Oxford (1964), p. 81.
F. A. L. Anet and C. R. Eves, Can. J. Chem.,36, 902 (1958).
E. Godar and R. P. Martella, J. Am. Chem. Soc.,79, 1402 (1957).
A. I. Gurevich, M. N. Kolosov, and V. G. Korobko, Zh. Organ. Khim.,6, 311 (1970).
N. Bhacca and D. Williams, Applications of NMR Spectroscopy in Organic Chemistry, Holden-Day, San Francisco (1964).
J. E. Blackmood, C. L. Gladys, K. L. Loening, A. E. Petrarca, and J. E. Rush, J. Am. Chem. Soc.,90, 509 (1968).
J. H. Brewster, Tetrahedron,13, 106 (1961).
N. Harada, M. Ohashi, and K. Nakanishi, J. Am. Chem. Soc.,90, 7349 (1968).
R. Willstätter and E. Waldschmidt-Leitz, Ber.,54, 113 (1921).
C. A. Brown and H. C. Brown, J. Am. Chem. Soc.,85, 1003 (1963).
Y. Kono, S. Takeuchi, H. Yonehara, F. Marumo, and Y. Saito, J. Antibiotics, Ser. A (in press) (1971).
Additional information
M. M. Shemyakin Institute of the Chemistry of Natural Compounds, Academy of Sciences of the USSR. Translated from Khimiya Prirodnykh Soedinenii, No. 1, pp. 104–112, January, 1971.
Rights and permissions
About this article
Cite this article
Gurevich, A.I., Kolosov, M.N., Korobko, V.G. et al. Structure and stereochemistry of the antibiotic abikoviromycin. Chem Nat Compd 7, 92–98 (1971). https://doi.org/10.1007/BF01032036
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01032036