Abstract
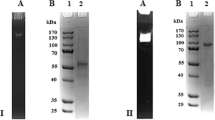
An enzyme capable of cleaving catechin was present in the mycelium ofCheatomium cupreum. Maximum synthesis of the enzyme occurred after 15 days growth. Sucrose and maltose increased enzyme synthesis among the carbon sources tested. Catechol, protocatechuic acid and phloroglucinol carboxylic acid were the intermediates of catechin degradation.Cheatomium cupreum containedmeta-cleaving enzymes for catechol and protocatechuic acid metabolism. Pyruvate was identified as an end-product. Catechin oxygenase from the mycelium ofC. cupreum was purified to homogeneity. It was optimum at pH 7.0 and 50°C and was highly specific for catechin, with a Km of 4 μm. Its molecular size was 40 kDa, as determined by gel filtration and gel electrophoresis, and it had a pI of 9.1.p-Chloromercuric benzoate, iodoacetate, N-ethylmaleimide, 2,2′-dipyridyl and EDTA markedly inhibited the enzyme activity. It was a glycoprotein.
Similar content being viewed by others
References
Adachi, K., Iwagawa, Y., Tanioka, H. & Takeda, Y. 1966 Purification and properties of homogentisate oxygenase fromPseudomonas fluorescens.Biochimica et Biophysica Acta 118, 88–97.
Aoki, K., Shinke, E. & Nishira, H. 1976 Chemical composition and molecular weight of yeast tannase.Agricultural and Biological Chemistry 40, 86–91.
Arunakumari, A. & Mahadevan, A. 1984 Utilization of aromatic substances byPseudomonas solanacearum.Indian Journal of Experimental Biology 22, 32–36.
Barth, G., Records, R., Bunnenberg, E. & Djerassi, C. 1971 Magnetic circular dichroism studies. XII. The determination of tryptophan in proteins.Journal of American Chemical Society 93, 2545–2547.
Beechy, R.B. & Ribbons, D.W. 1972 Oxygen uptake measurement.Methods in Microbiology 6b, 25–53.
Cambie, R.C. 1959 The extractives ofDysoxylum spectalile, Hook. Journal of the Chemical Society 468–469.
Chandra, T., Madhavakrishna, W. & Nayudamma, Y. 1969 Astringency in fruits. I. Microbial degradation of catechin.Canadian Journal of Microbiology 15, 303–306.
Chandrakantha, A.A., Krishnamurthy, V., Madhavakrishna, W. & Nayudamma, Y. 1973 Astringency in fruits. VI. Microbial degradation of cashew apple (Anacardium occidentale) tannin.Leather Science 20, 337–342.
Dawson, R.M.C., Elliot, D.C., Elliot, W.H. & Jones, K.M. 1986 Detection of biochemical compounds. InData for Biochemical Research, eds Dawson, R.M.C., Elliot, D.C., Elliot, W.H. & Jones, K.M. p. 466 Oxford: Clarendon Press.
Galiotou-Panayotou, M. & Macris, B.J. 1986 Degradation of condensed tannin byCalvatia gigantea.Applied Microbiology and Biotechnology 23, 502–506.
Galiotou-Panayotou, M., Rodis, P., Macris, B.J. & Stathakos, D. 1988 Purification of a novel enzyme involved in catechin degradation byCalvatia gigantea.Applied Microbiology and Biotechnology 28, 543–545.
Gauthier, J.J. & Rittenberg, S.C. 1971 Metabolism of nicotinic acid. I. Purification and properties of 2,5-dihydroxy pyridine oxygenase fromPseudomonas putida N-9.Journal of Biological Chemistry 246, 3737–3742.
Hayaishi, O. 1974 Nomenclature, classification and general properties of oxygenases. InMolecular Mechanisms of Oxygen Activation, ed Hayaishi, O. pp. 6–10, New York: Academic Press.
Hayaishi, O., Katagiri, M. & Rothberg, S. 1957 Studies on oxygenases. Pyrocatechase.Journal of Biological Chemistry 229, 905–920.
Hegeman, G.D. 1966a Synthesis of the enzymes of the mandalate pathway byPseudomonas putida. I. Synthesis of enzymes by wild type.Journal of Bacteriology 91, 1140–1154.
Hegeman, G.D. 1966b Synthesis of the enzymes of the mandalate pathway ofPseudomonas putida. II. Isolation and properties of blocked mutants.Journal of Bacteriology 91, 1155–1160.
Jeffrey, A.M., Knight, M. & Evans, W.C. 1969 The bacterial metabolism of flavonoids: Hydroxylation of taxifolin.Journal of General Microbiology 56, i.
Jeffrey, A.M., Knight, M. & Evans, W.C. 1972 The bacterial degradation of flavonoids.Biochemical Journal 130, 373–381.
Kaneda, N., Sato, M. & Yagi, K. 1982 Analysis of dansyl amino acids by reverse phase high performance liquid chromatography.Analytical Biochemistry 127, 49–54.
Lewis, J.A. & Starkey, R.L. 1968 Vegetable tannin and their decomposition and effects on decomposition of organic compounds.Soil Science 106, 241–247.
Mahadevan, A., Sambandam, T. & Sivaswamy, N. 1982 Microbial degradation of phenolic substances.Indian Review of Life Sciences 2, 1–18.
Mahadevan, A. & Sridhar, R. (eds) 1986Methods in Physiological Plant Pathology. Madras: Sivakami Publications.
Mason, H. S. 1949 The chemistry of melanin. VI. Mechanism of the oxidation of catechol by tyrosinase.Journal of Biological Chemistry 181, 803–812.
Muthukumar, G., Arunakumari, A. & Mahadevan, A. 1982 Degradation of aromatic compounds byRhizobium spp.Plant and Soil 69, 163–169.
Myers, W.F. & Huang, K.Y. 1966 Separation of intermediates of the citric acid cycle and related compounds by thin layer chromatography.Analytical Biochemistry 17, 210–215.
Nakai, C., Kakamiyama, H., Saeki, Y. & Nozaki, M. 1979 Nonidentical subunits of pyrocatechase fromPseudomonas arvilla C-1.Archives of Biochemistry and Biophysics 195, 12–22.
Oka, T. & Simpson, F.J. 1971 Quercetinase, a dioxygenase containing copper.Biochemical and Biophysical Research Communications 43, 1–5.
Peterson, G.L. 1979 Review of the Folin phenol quantitation method of Lowry, Rosebrough, Farr and Randall.Analytical Biochemistry 100, 201–220.
Rajagopalan, K.V. & Handler, P. 1964 The absorption spectra of iron-flavoproteins.Journal of Biological Chemistry 239, 192–205.
Reddy, C.C. & Vaidyanathan, C.S. 1975 Purification, properties and induction of a specific benzoate-4-hydroxylase fromAspergillus niger (UBC 814).Biochimica et Biophysica Acta 384, 46–57.
Roux, D.G., Miller, T.H. & Maihs, E.A. 1961 An improved lead salt method for purifying black wattle tannin.Journal of American Leather Chemists' Association 56, 362–364.
Sedlick, J. & Lindsay, R.H. 1968 Estimation of total, protein bound and non-protein sulphydryl groups in tissues with Ellman's reagent.Analytical Biochemistry 25, 192–205.
Segrest, J.P. & Jackson, R.L. 1972 Molecular weight determination of glycoproteins by polyacrylamide gel electrophoresis on sodium dodecyl sulphate.Methods in Enzymology 28, 54–63.
Simpson, R.J., Neuberger, M.R. & Linct, Y. 1976 Complete amino acid analysis of a protein from a single hydrolysate.Journal of Biological Chemistry 251, 1936–1940.
Spiess, J. 1986 Carboxy terminal by modified Edman degradation. InMicrostructural Studies in Peptide and Protein Chemistry, ed Shivley, J.E. pp. 363–377. New Jersey: Humanan Publications.
Sze, I.S.Y. & Dagley, A. 1984 Properties of salicylate hydrooxylase and hydroxyquinol 1,2-dioxygenase purified fromTrichosporon cutaneum.Journal of Bacteriology 159, 353–359.
Updegraff, D.M. & Grant, W.D. 1975 Microbial utilization ofPinus radiata bark.Applied Microbiology 30, 722–726.
Vega, R.R., Corsini, D. & LeTourneau, D. 1970 Nonvolatile organic acids produced bySclerotinia sclerotium in synthetic liquid media.Mycologia 62, 332–338.
Weber, K. & Osborn, M. 1969 The reliability of molecular weight determinations by sodium dodecyl sulphate-polyacrylamide gel electrophoresis.Journal of Biological Chemistry 244, 4406–4412.
William, F. & Mahadevan, A. 1980 Degradation of aromatic compounds byXanthomonas species.Journal of Plant Diseases and Protection 87, 738–744.
Yoshida, R., Hori, K., Puriware, M., Saeki, Y., Kagamiyama, H. & Nozaki, M. 1976 Nonidentical subunits of protocatechuate 3,4-dioxygenase.Biochemistry 15, 4048–4053.
Additional information
T. Sambandam was and A. Mahedevan is with the Center for Advanced Studies in Botany, University of Madras, Guindy Campus, Madras-600025, India. T. Sambandam is now with the Department of Medicine, University of Alabama at Birmingham, Birmingham, AL 35294, USA.
Rights and permissions
About this article
Cite this article
Sambandam, T., Mahadevan, A. Degradation of catechin and purification and partial characterization of catechin oxygenase fromChaetomium cupreum . World J Microbiol Biotechnol 9, 37–44 (1993). https://doi.org/10.1007/BF00656513
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00656513