Abstract
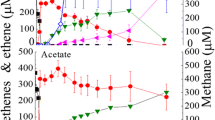
Methanogenesis from acetate by a rod-shaped enrichment culture grown at 60° C was found to require the presence of two organisms rather than a single aceticlastic methanogen. A thermophilic Methanobacterium which grew on H2/CO2 or formate was isolated from the enrichment. Lawns of this methanogen were used to co-isolate an “acetate oxidizer” in roll tubes containing acetate agar. The rod-shaped acetate oxidizer was morphologically distinct from the methanogen and did not show F420 autofluorescence. The coculture completely degraded 40 μmol/ml acetate, and produced nearly equal quantities of methane, and methanogenesis was coupled with growth. The doubling time for the coculture at 60°C was 30–40 h and the yield was 2.7±0.3 g dry wt/mol CH4. Studies with 14C-labelled substrates showed that the methyl group and the carboxyl group of acetate were both converted primarily to CO2 by the coculture and that CO2 was concurrently reduced to CH4. During growth, there was significant isotopic exchange between CO2 and acetate, especially with thecarboxyl position of acetate. These results support a mechanism for methanogenesis from acetate by the coculture in which acetate was oxidized to CO2 and H2 by one organism, while H2 was subsequently used by a second organism to reduce CO2 to CH4. Since the H2 partial pressure must be maintained below 10-4 atm by the methanogen for acetate oxidation to be thermodynamically feasible, this is an example of obligate interspecies hydrogen transfer. This mechanism was originally proposed for a single organism by Barker in 1936.
Similar content being viewed by others
References
Balch WE, Fox GE, Magrum LJ, Woese CR, Wolfe RS (1979) Methanogens: reevaluation of a unique biological group. Microbiol Rev 43:260–296
Barker HA (1936) On the biochemistry of the methane fermentation. Arch Mikrobiol 7:404–419
Barker HA, Ruben S, Kamen MD (1940) The reduction of radioactive carbon dioxide by methane-producing bacteria. Proc Natl Acad Sci USA 26:426–430
Boone DR, Bryant MP (1980) Propionate-degrading bacterium, Syntrophobacter wolinii sp. nov. gen. nov., from methanogenic ecosystems. Appl Environ Microbiol 40:626–632
Brock TD, O'Dea K (1977) Amorphous ferrous sulfide as a reducing agent for culture of anaerobes. Appl Environ Microbiol 33:254–257
Bryant MP (1979) Microbial methane production-theoretical aspects. J Anim Sci 48:193–201
Bryant MP, Wolin EA, Wolin MJ, Wolfe RS (1967) Methanobacillus omelianskii, a symbiotic association of two species of bacteria. Arch Mikrobiol 59:20–31
Bryant MP, Campbell LL, Reddy CA, Crabhill MR (1977) Growth of Desulfovibrio on lactate or ethanol media low in sulfate in association with hydrogen-utilizing methanogenic bacteria. Appl Environ Microbiol 33:1162–1169
Buswell AM, Sollo FW Jr (1948) The mechanism of the methane fermentation. J Am Chem Soc 70:1778–1780
Chen M, Wolin MJ (1977) Influence of CH4 production by Methanobacterium ruminantium on the fermentation of glucose and lactate by Selenomonas ruminantium. Appl Environ Microbiol 34:756–759
Claypool GE, Kaplan IR (1974) The origin and distribution of methane in marine sediments. In: Kaplan IR (ed) Natural gases in marine sediments. Plenum Press, New York, pp 99–140
Coolhaas VC (1928) Zur Kenntnis der Dissimilation fettsaurer Salze und Kohlenhydrate durch thermophile Bakterien. Zbl Bakteriol Parasitenkd Infektionskr Hyg Abt 2 75:161–170
Doddema HJ, Vogels GD (1978) Improved identification of methanogenic bacteria by fluorescence microscopy. Appl Environ Microbiol 36:752–754
Ehrlich GG, Goerlitz DF, Bourell JH, Eisen GV, Godsy EM (1981) Liquid chromatographic procedure for fermentation product analysis in the identification of anaerobic bacteria. Appl Environ Microbiol 42:878–885
Fuchs G, Stupperich E (1980) Acetyl CoA, a central intermediate of autotrophic CO2 fixation in Methanobacterium thermoautotrophicum. Arch Microbiol 127:267–272
Hippe H, Caspari D, Fiebig K, Gottschalk G (1979) Utilization of trimethylamine and other N-methyl compounds for growth and methane formation by Methanosarcina barkeri. Proc Natl Acad Sci USA 76:494–498
Krzycki JA, Wolkin RH, Zeikus JG (1982) Comparison of unitrophic and mixotrophic substrate metabolism by an acetate-adapted strain of Methanosarcina barkeri. J Bacteriol 149: 247–254
Lehninger AL (1975) Biochemistry, 2nd edn. Worth, Inc., New York
Leigh JA, Mayer F, Wolfe RS (1981) Acetogenium kivui, a new thermophilic hydrogen-oxidizing acetogenic bacterium. Arch Microbiol 129:275–280
Ljungdahl LG, Wood HG (1969) Total synthesis of acetate from CO2 by heterotrophic bacteria. Ann Rev Microbiol 23: 515–538
Lovley DJ, Klug MJ (1982) Intermediary metabolism of organic matter in the sediments of a eutrophic lake. Appl Environ Microbiol 43:552–560
Mackie RI, Bryant MP (1981) Metabolic activity of fatty acid-oxidizing bacteria and the contribution of acetate, propionate, butyrate, and CO2 to methanogenesis in cattle waste at 40 and 60°C. Appl Environ Microbiol 41:1363–1373
Mah RA (1980) Isolation and characterization of Methanococcus mazei. Curr Microbiol 3:321–326
Mah RA, Ward DM, Baresi L, Glass TL (1977) Biogenesis of methane. Ann Rev Microbiol 31:309–341
Mah RA, Smith MR, Baresi L (1978) Studies on an acetate fermenting strain of Methanosarcina. Appl Environ Microbiol 35:1174–1184
Marty DG, Bianchi AJM (1981) Isolement de deux souches methanogènes thermophiles appartenant au genre Methanobacterium. CR Acad Sci Paris 292:41–43
McInerney MJ, Bryant MP (1980) Syntrophic associations of H2-utilizing methanogenic bacteria and H2-producing alcohol and fatty acid degrading bacteria in anaerobic degradation of organic matter. In: Gottschalk G, Pfennig N, Werner H (eds) Anaerobes and anaerobic infections. Fischer, Stuttgart New York, pp 117–126
McInerney MJ, Bryant MP, Pfennig N (1979) Anaerobic bacterium that degrades fatty acids in syntrophic association with methanogens. Arch Microbiol 122:129–135
McInerney MJ, Bryant MP, Hespell RB, Costerton JW (1981) Syntrophomonas wolfei gen. nov. sp. nov., an anaerobic, syntrophic, fatty acid-oxidizing bacterium. Appl Environ Microbiol 41:1029–1039
Mountfort DO, Asher RA (1978) Changes in proportion of acetate and carbon dioxide used as methane precursors during the anaerobic digestion of bovine waste. Appl Environ Microbiol 35:648–654
Mountfort DO, Asher RA, Mays EL, Tiedje JM (1980) Carbon and electron flow in mud and sandflat intertidal sediments at Delaware Inlet, Nelson, New Zealand. Appl Environ Microbiol 39:686–694
Nelson DR, Zeikus JG (1974) Rapid method for the radioisotopic analysis of gaseous end products of anaerobic metabolism. Appl Microbiol 28:258–261
Robinson JA, Tiedje JM (1982) Kinetics of hydrogen consumption by rumen fluid, anaerobic digestor sludge and sediment. Appl Environ Microbiol 44:1374–1384
Sansone FJ, Martens CS (1981) Methane production from acetate and associated methane fluxes from anoxic coastal sediments. Science 211:707–709
Schauer NL, Brown DP, Ferry JG (1982) Kinetics of formate metabolism in Methanobacterium formicium and Methanospirillum hungatei. Appl Environ Microbiol 44:549–554
Schonheit P, Moll J, Thauer RK (1980) Growth parameters (K s, μmax, Y s) of Methanobacterium thermoautotrophicum. Arch Microbiol 127:59–65
Smith MR, Mah RA (1980) Acetate as sole carbon and energy source for growth of Methanosarcina Strain 227. Appl Environ Microbiol 39:993–999
Smith MR, Zinder SH, Mah RA (1980) Microbial methanogenesis from acetate. Proc Biochem 15:34–39
Smith PH, Mah RA (1966) Kinetics of acetate metabolism during sludge digestion. Appl Microbiol 14:368–371
Soehngen NL (1910) Sur le role du methane dans la vie organique. Recueil des Travaux Chimiques 29:238–274
Stadtman TC, Barker HA (1949) Studies on the methane fermentation. VII. Tracer experiments on the mechanism of methane formation. Arch Biochem 21:256–264
Strayer RF, Tiedje JM (1978) Kinetic parameters of the conversions of methane precursors to methane in a hypereutrophic lake sediment. Appl Environ Microbiol 36:330–340
Thauer RK, Jungermann K, Decker K (1977) Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev 41:100–180
Warford AL, Kosiur DR, Doose PR (1979) Methane production in Santa Barbara basin sediments. Geomicrobiol J 1:117–137
Weimer PJ, Zeikus JG (1977) Fermentation of cellulose and cellobiose by Clostridium thermocellum in the absence and presence of Methanobacterium thermoautotrophicum. Appl Environ Microbiol 33:289–297
Weimer PJ, Zeikus JG (1978) Acetate metabolism in Methanosarcina barkeri. Arch Microbiol 119:175–182
Winfrey MR, Zeikus JG (1979) Anaerobic metabolism of immediate methane precursors in Lake Mendota. Appl Environ Microbiol 37:244–253
Winter J, Wolfe RS (1979) Complete degradation of carbohydrate to carbon dioxide and methane by syntrophic cultures of Archaebacterium woodii and Methanosarcina barkeri. Arch Microbiol 121:97–102
Winter JU, Wolfe RS (1980) Methane formation from fructose by syntrophic associations of Acetobacterium woodii and different strains of methanogens. Arch Microbiol 124:73–79
Wolin MJ, Miller TL (1982) Interspecies hydrogen transfer: 15 years later. ASM News 48:561–565
Zehnder AJB, Huser BA, Brock TD, Wuhrmann K (1980) Characterization of an acetate-decarboxylating, non-hydrogen-oxidizing methane bacterium. Arch Microbiol 124:1–11
Zeikus JG (1977) The biology of methanogenic bacteria. Bacteriol Rev 41:514–541
Zeikus JG, Wolfe RS (1972) Methanobacterium thermoautotrophicus sp. nov., an anaerobic, autotrophic, extreme thermophile. J Bacteriol 109:707–713
Zeikus JG, Weimer PJ, Nelson DR, Daniels L (1975) Bacterial methanogenesis: acetate as a methane precursor in pure culture. Arch Microbiol 104:129–134
Zeikus JG, Ben-Bassat A, Hegge PW (1980) Microbiology of methanogenesis in thermal, volcanic environments. J Bacteriol 143:432–440
Zinder SH, Mah RA (1979) Isolation and characterization of a thermophilic strain of Methanosarcina unable to use H2−CO2 for methanogenesis. Appl Environ Microbiol 38:996–1008
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zinder, S.H., Koch, M. Non-aceticlastic methanogenesis from acetate: acetate oxidation by a thermophilic syntrophic coculture. Arch. Microbiol. 138, 263–272 (1984). https://doi.org/10.1007/BF00402133
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00402133