Abstract
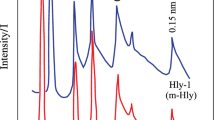
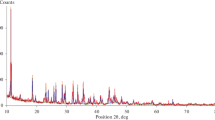
The decomposition reaction of kaolinite has been investigated as a function of the defectivity of the starting material and the temperature of reaction. Time resolved energy-dispersive powder diffraction patterns have been measured using synchrotron radiation, both under a constant heating rate (heating rates from 10 to 100° C/min) and in isothermal conditions (in the temperature range 500 to 700° C). The apparent activation energy of the dehydroxylation process is different for kaolinites exhibiting a different degree of stacking fault density. The results of the analysis of the kinetic data indicate that the starting reaction mechanism is controlled by diffusion in the kaolinite particle. The diffusion process is dependent on the defective nature of both kaolinite and metakaolinite. At high temperatures, and at higher heating rates, the reaction mechanism changes and the resistance in the boundary layer outside the crystallites becomes the rate-limiting factor, and nucleation begins within the reacting particle. During the final stage of the dehydroxylation process the reaction is limited by heat or mass transfer, and this might be interpreted by the limited diffusion between the unreacted kaolinite domains and the metakaolinite matrix.
Similar content being viewed by others
References
Artioli G, Bellotto M, Gualtieri A, Pavese A (1994) Nature of structural disorder in natural kaolinites: a new model based on computer simulations of powder diffraction data and electrostatic energy calculations. Clays Clay Minerals (submitted for publication)
Bamford CH, Tipper CFH (1980) Comprehensive chemical kinetics. Elsevier, New York, 22:41–113
Brindley GW, Nakahira M (1957a) Role of water vapor in the dehydroxylation of clay minerals. Clay Minerals Bull 3:114–119
Brindley GW, Nakahira M (1957b) Kinetics of dehydroxylation of kaolinite and halloysite. J Am Ceram Soc 40:346–350
Brindley GW, Sharp JH, Patterson JH, Narahari BN (1967) Kinetics and mechanism of dehydroxylation processes. I. Temperature and vapor pressure dependence of dehydroxylation of kaolinite. Am Mineral 52:201–211
Clark SM (1989) Energy-dispersive powder diffraction at the SRS. Nucl Inst Meth Phys Res A276:381–387
Criado JM, Ortega A, Real C, Torres de Torres E (1984) Reexamination of the kinetics of the thermal dehydroxylation of kaolinite. Clay Minerals 19:653–661
Donnay G, Wyart J, Sabatier G (1959) Structural mechanisms of thermal and compositional transformations in silicates. Z Kristallogr 112:161–168
Fripiat JJ, Toussaint F (1963) Dehydroxylation of kaolinite. II. Conductometric measurements and infrared spectroscopy. J Phys Chem 67:30–36
Goldsmith JR (1987) Al/Si interdiffusion in albite: effect of pressure and the role of hydrogen. Contrib Mineral Petrol 95:311–321
Goldsmith JR (1988) Enhanced Al/Si diffusion in KAlSi3O8 at high pressures: the effect of hydrogen. J Geology 96:109–124
Goldsmith JR, Jenkins DM (1985) The high-low albite relations revealed by reversal of degree of order at high pressures. Am Mineral 70:911–923
Grimshaw RW, Heaton E, Roberts AL (1945) Refractory clays. Trans Brit Ceram Soc 44:69–92
Hancock JD, Sharp JH (1972) Method of comparing solid-state kinetic data and its application to the decomposition of kaolinite, brucite, and BaCO3. J Am Ceram Soc 55:74–77
Holt JB, Cutler IB, Wadsworth ME (1962) Rate of thermal dehydration of kaolinite in vacuum. J Am Ceram Soc 45:133–136
Johnson HB, Kessler F (1969) Kaolinite dehydroxylation kinetics. J Am Ceram Soc 52:199–204
Leonard AJ (1977) Structural analysis of the transition phases in the kaolinite-mullite thermal sequence. J Am Ceram Soc 60:37–43
Levenspiel O (1973) Chemical reaction engineering. John Wiley, New York
MacKenzie KJD, Brown IWM, Meinhold RH, Bowden ME (1985) Outstanding problems in the kaolinite-mullite reaction sequence investigated by 29Si and 27Al solid-state nuclear magnetic resonance: I, metakaolinite. J Am Ceram Soc 68:293–297
McIlvried HG, Massoth FE (1973) Effect of particle size distribution on gas-solid reaction kinetics for spherical particles. Ind Eng Chem Fundam 12:225–229
Murray P, White J (1949) Kinetics of thermal dehydration of clays. Trans Brit Ceram Soc 48:187–206
Murray P, White J (1955a) Kinetics of thermal dehydration characteristics of the clay minerals: I. Trans Brit Ceram Soc 54:137–150
Murray P, White J (1955b) Kinetics of thermal dehydration characteristics of the clay minerals: II. Trans Brit Ceram Soc 54:151–187
Murray P, White J (1955c) Kinetics of thermal dehydration characteristics of the clay minerals: III. Trans Brit Ceram Soc 54:204–238
Ortega A, Rouquerol F, Akhouayri S, Laureiro Y, Rouquerol J (1993) Kinetical study of the thermolysis of kaolinite between 30 and 1000° C by controlled rate evolved gas analysis. Appl Clay Sci 8:207–214
Redfern SAT (1987) The kinetics of dehydroxylation of kaolinite. Clay Minerals 22:447–456
Salje EKH (1990) Phase transitions in ferroelastic and co-elastic crystals. Cambridge University Press, Cambridge, p 202–211
Suitch PR (1986) Mechanism for the dehydroxylation of kaolinite, dikite, and nacrite from room temperature to 455° C. J Am Ceram Soc 69:61–65
Toussaint F, Fripiat JJ, Gastuche MC (1963) Dehydroxylation of kaolinite. I. Kinetics. J Phys Chem 67:26–30
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bellotto, M., Gualtieri, A., Artioli, G. et al. Kinetic study of the kaolinite-mullite reaction sequence. Part I: Kaolinite dehydroxylation. Phys Chem Minerals 22, 207–217 (1995). https://doi.org/10.1007/BF00202253
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00202253